Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Pulmonary Human Cardiac Hemi Artery Equipment Supplied In Usa
57 equipment items found
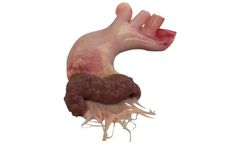
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
The Ideal Patch for: Congenital reconstructions. Repair of the RVOT. ...
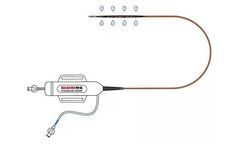
Manufactured by:Thrombolex, Inc. based inNew Britain, PENNSYLVANIA (USA)
The BASHIR N-X Endovascular Catheter (BASHIR™ N-X) is a device intended for the localized infusion of physician specified fluids, into the peripheral vasculature, including the pulmonary arteries. ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
Femoral Vein vs. PTFE Graft: Stage I Norwood1. For patients receiving a Femoral Vein: Better growth and development of branch pulmonary arteries. Improved Nakata Index. No recognizable adverse events with ...
Manufactured by:Medical Positioning, Inc. (MPI) based inKansas City, KANSAS (USA)
Patients easily achieve steady-state exercise with this cycle ergometer, allowing for accurate intracardiac hemodynamic assessment during nuclear studies and right heart ...
Manufactured by:Medical Positioning, Inc. (MPI) based inKansas City, KANSAS (USA)
Patients easily achieve steady-state exercise with this cycle ergometer, allowing for accurate intracardiac hemodynamic assessment during nuclear studies and right heart catheterization. We designed the Cath Ergometer® for use with cath or nuclear tables. Technologists can custom fit and adjust this cycle ergometer from patient to patient for right heart catheterizations. The cycle ergometer ...
Manufactured by:LeMaitre Vascular, Inc. based inBurlington, MASSACHUSETTS (USA)
RestoreFlow Allografts provide a high quality allograft tissue using state-of-the-art technologies for tissue restoration and preparation techniques. These allografts can be used for cardiac repair and reconstruction, as well as for adults with extensive valvular disease. Durable Alternative Valves and ...
Manufactured by:Endotronix, Inc. based inLisle, ILLINOIS (USA)
Introducing the Cordelia® System, a solution that helps you achieve guideline-directed medical therapy with your patients using a streamlined workflow. Along with regular clinic visits, the system uses an intuitive Patient Management Portal and an at-home patient kit to improve outcomes and proactively treat more ...
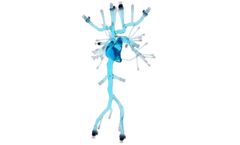
Manufactured by:United Biologics, Inc. based inIrvine, CALIFORNIA (USA)
Designed for Emerging Pulmonary Segment: Developed from DICOM Data, ensuring clinical accuracy when navigating the Right Atrium, Right Ventricle & Pulmonary Arteries. Lifelike Pulmonary Embolism (PE) & Deep Vein Thrombosis (DVT) ...
Manufactured by:Lonza Group Ltd based inBasel, SWITZERLAND
Culture system containing EBMTM-2 Basal Medium (CC-3156) and EGMTM-2 SingleQuotsTM Supplements (CC-4176) required for growth of Endothelial Cells. Available for immediate ...
Manufactured by:Lonza Group Ltd based inBasel, SWITZERLAND
Culture system containing EBMTM-2 Basal Medium (CC-3156) and EGMTM-2 MV Microvascular Endothelial Cell Growth Medium SingleQuotsTM supplements (CC-4147) required for growth of Microvascular Endothelial ...
Manufactured by:Abbott Laboratories based inAbbott Park, ILLINOIS (USA)
Prevent Heart Failure Hospitalizations by Remotely Managing PA Pressure with the CardioMEMS HF ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
Applications - Tissue: Descending Thoracic Aorta. Size: Diameter = 14mm (min.). Catalog Number: A0203. Additional CryoGraft Tissue. Ideal for: Conduit repair, Aortic graft infections, Aneurysm ...
Manufactured by:LeMaitre Vascular, Inc. based inBurlington, MASSACHUSETTS (USA)
CardioCel is an acellular, strong, pliable collagen bioscaffold with optimized biocompatibility and zero aldehyde toxicity. The collagen bioscaffold allows for transformative repair with harmonious healing, providing long-term durability that enables native cells to successfully grow and differentiate through the entire ...
by:Abiomed Heart Recovery based inDanvers, MASSACHUSETTS (USA)
The only percutaneous pump approved for right heart support with single vascular access, designed for intelligent patient management. Impella RP with SmartAssist is the only right heart pump equipped with dual-sensor technology to assist with pump management. This technology pumps blood from the inferior vena cava to the pulmonary artery. The pump is inserted with venous access and advanced over ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
Orenitram is a prostacyclin mimetic indicated for treatment of pulmonary arterial hypertension (PAH; WHO Group 1), to delay disease progression and to improve exercise capacity. The studies that established effectiveness included predominately patients with WHO functional class II-III symptoms and etiologies of idiopathic or heritable PAH (66%) or PAH associated with connective tissue disease ...
by:Vivonics Inc. based inBedford, MASSACHUSETTS (USA)
Congenital heart disease (CHD) refers to the various malformations of the heart and surrounding vessels that occur prior to birth. Almost 1% of children are born with some form of significant CHD, and CHD remains a leading cause of infant death in the United States. Many infants with CHD develop exuberant blood flow to their lungs that, if left untreated, will result in overwhelming congestive ...
Manufactured by:NaviGate Cardiac Structures, Inc., (NCSI) based inLake Forest, CALIFORNIA (USA)
The GATE™ System is intended to treat functional tricuspid regurgitation (FTR)/tricuspid insufficiency by replacing the function of a patient’s dysfunctional native tricuspid valve with a bio-prosthetic atrioventricular valved stent (AVS). The AVS is designed to restore valve function in patients presenting with FTR/tricuspid insufficiency without the need for conventional, open-heart ...
Manufactured by:Medtronic based inMinneapolis, MINNESOTA (USA)
The Crescent™* catheter is the first FDA-cleared, jugular dual lumen long-term ECMO catheter. It allows for more accurate placement with just one cannulation site, delivers enhanced flow dynamics, and helps maintain optimal flow† once placed. ...
Manufactured by:Renata Medical based inNewport Beach, CALIFORNIA (USA)
The Minima Stent is designed for use in the treatment of native or acquired pulmonary artery stenoses or coarctation of the aorta. The stent comes packaged on a specifically designed delivery system which allows for sheath-less delivery to the target ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Seralutinib (GB002) is an inhaled PDGFRα and PDGFRβ inhibitor. Seralutinib also targets to CSF1R and c-KIT with IC50s of 8 nM and 14 nM, respectively. Seralutinib (GB002) is used in the study for pulmonary arterial ...