Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Rubella Equipment Supplied In Usa
6 equipment items found
by:Diasorin based inSALUGGIA, ITALY
The LIAISON® Rubella IgG assay uses chemiluminescence immunoassay (CLIA) technology for the quantitative determination of specific IgG antibodies to rubella virus in human serum or plasma samples. The test has to be performed on the LIAISON® analyzer family. ...
Manufactured by:Arlington Scientific, Inc. based inSpringville, UTAH (USA)
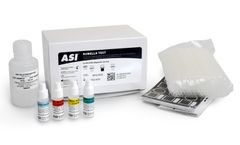
The ASI Rubella test (also known as German Measles) is a convenient and specific rapid latex particle agglutination test for the Qualitative and Semiquantitative determination of rubella virus antibodies in serum. The kit can be used to test diluted or undiluted samples. The test aids in the diagnosis of recent or active rubella infection and in ...
Manufactured by:ZEUS Scientific, Inc. based inBranchburg, NEW JERSEY (USA)
The ZEUS AtheNA Multi-Lyte ToRCH IgG Plus Test System is intended for the qualitative detection of IgG class antibodies to Toxoplasma gondii, Rubella, Cytomegalovirus and Herpes Simplex 1 and 2 viruses in human serum. The test system is intended to be used as an aid in the diagnosis of diseases caused by the exposure to Toxoplasma gondii, Rubella, Cytomegalovirus ...
Manufactured by:Arlington Scientific, Inc. based inSpringville, UTAH (USA)
Arlington Scientific, Inc.™ (ASI™) offers a wide range of bulk latex serology reagents ready to meet the needs of small to large companies seeking to enhance their current product or develop new product lines. ASI reagents are currently exported to Europe, Latin America and the Pacific Rim. ASI's products are developed and produced with unique formulations using state of the art ...
Manufactured by:Accel Diagnostics based inHouston, TEXAS (USA)
A Rapid Test for the Quantitation of the Titer of Antibody Against the Spike Protein of the SARS-COV-2 VIRUS. Accel Diagnostics’ rapid quantitative COVID-19 serology assay for the detection and qualification anti-Spike SARS-COV-2 antibody concentration (IgG) is now ...
Manufactured by:VEO Ophthalmics, LLC based inCincinnati, OHIO (USA)
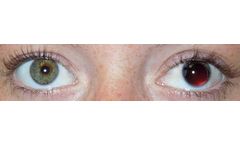
The CUSTOMFLEX® ARTIFICIALIRIS is intended for use as an iris prosthesis for the treatment of iris defects. The CUSTOMFLEX® ARTIFICIALIRIS is indicated for use in children and adults for the treatment of full or partial aniridia resulting from congenital aniridia, acquired defects, or other conditions associated with full or partial aniridia. When implanted, the CUSTOMFLEX® ...