Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Surgical Implant Equipment Supplied In Usa
68 equipment items found
Manufactured by:Voyage Medical Solutions based inLongview, TEXAS (USA)
Our VMS team has created partnerships with multiple surgical device companies for our surgeons’ needs. Contact us today for a complete portfolio. If you don’t see a specific implant product you’re looking for, feel free to contact us to see if we can get you the durable medical equipment you ...
Manufactured by:enVVeno Medical based inIrvine, CALIFORNIA (USA)
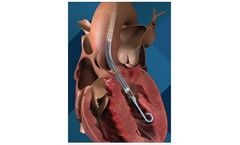
The VenoValve is a first-in-class surgically implanted solution being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). Implanted into the femoral vein, the VenoValve is designed to act as a one-way valve to help restore proper blood flow up the leg, to return sufficient blood back to the heart. ...
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
Impella 5.0 is a temporary, minimally invasive heart pump that provides circulatory support, enabling the heart to rest and recover. Impella LD is a surgically implanted heart pump that provides temporary support to the heart during and after a procedure. Impella 5.0 and Impella LD heart pumps allow patients to walk around while on support to improve recovery, if ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Non-bone tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. Non-bone tendons used in ACL reconstruction procedures allow for multiple fixation ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's bone-tendon-bone (BTB) grafts are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These grafts allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Matrix HD Allograft Dermis is an acellular human dermis allograft sterilized to a Sterility Assurance Level (SAL) of 10-6 using the Tutoplast Tissue Sterilization Process. This proprietary process retains the three-dimensional intertwined multidirectional fibers and mechanical properties of the native dermis tissue. The Matrix HD graft provides a natural scaffold to support the body's ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's Achilles tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These tendons allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Conventional allografts are used for various procedures to fill bony voids or gaps in a patient's skeletal system, restore segmental bone loss due to trauma, osteoporosis, removal of tumors or to aid in the surgical correction for a deformity. These grafts provide a scaffold for bone ingrowth to allow for remodeling with the patient’s own bone. These grafts are pre-cut to select sizes to ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical offers the only meniscal allograft with both bone and meniscus sterilized through the BioCleanse® Tissue Sterilization Process. The BioCleanse processed meniscus graft provides a sterilized alternative to traditional aseptically processed meniscus allografts and sets the standard for safety and ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical’s fresh-stored osteochondral (OC) allografts enable surgeons to resurface osteochondral defects with mature hyaline cartilage and repair subchondral bone in a single procedure. Fresh grafts are cleansed, processed and preserved to maintain chondrocyte ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Fortiva Porcine Dermis is a non-crosslinked acellular porcine dermal matrix offering a safe and natural biologic option. Fortiva implants are processed through the Tutoplast® Tissue Sterilization Process and terminally sterilized via validated low-dose gamma irradiation to achieve a sterility assurance level (SAL) of 10-6. Fortiva Porcine Dermis is a ready-to-use biologic implant that acts as ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Cortiva Allograft Dermis is a non-crosslinked acellular dermal matrix offering a safe and natural biologic option and sterilized to a sterility assurance level (SAL) of 10-6 through the Tutoplast® Tissue Sterilization ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Whole bones are used as a sterile source of cortical and cancellous bone. These implants may also be used in procedures that restore segmental and critical bone loss in the extremities due to trauma, bone disease, or from the removal of tumors and/or hardware. After being processed through RTI's BioCleanse® Tissue Sterilization Process, they are terminally sterilized in their final packaging ...
Manufactured by:TissX based inMinneapolis, MINNESOTA (USA)
The development of percutaneous cardiac valves (aortic, mitral, tricuspid and pulmonary). The development of surgically implanted cardiac valves. Pericardial Patches and other tissue for surgical, vascular and other applications. Functional components for structural heart disease treatment and other vascular uses. ...
by:Abiomed Heart Recovery based inDanvers, MASSACHUSETTS (USA)
Impella 5.5 with SmartAssist delivers full cardiac support with maximum unloading, allowing the heart to rest and recover. It is a microaxial, surgically implanted heart pump that unloads the left ventricle, reduces ventricular work, and provides the circulatory support necessary to allow recovery and early assessment of residual myocardial function. It is ...
Manufactured by:Axogen (AXGN) based inAlachua, TEXAS (USA)
Axoguard Nerve Protector is the only porcine submucosa extracellular (ECM) matrix surgical implant used to protect injured nerves and to reinforce nerve reconstruction while preventing soft tissue attachments. Designed to protect and isolate, the Axoguard multi-laminar ECM separates and protects the nerve from the surrounding tissues during the healing process. ...
Manufactured by:Precision Spine, Inc. based inParsippany, NEW JERSEY (USA)
The AccuFit® Lateral Plate System is indicated for use via a lateral or anterolateral surgical approach above the bifurcation of the great vessels in the treatment of thoracic and thoracolumbar (T1-L 5) spine instability. The AccuFit® Lateral Plate System consists of non-sterile, single use, titanium alloy (Ti-6AI-4V ELI per ASTM F136) rigid plates and bone screws of ...
Manufactured by:Narang Medical USA Corporation based inMiami, FLORIDA (USA)
This text outlines a comprehensive range of orthopedic surgical implants and instruments specialized for the distal radius. The primary focus is on Variable Angle Volar Distal Radius Safety Lock Plates measuring 2.4mm thickness, available with various configurations, such as 4, 5, or 6 head holes, to accommodate different surgical needs. ...
Manufactured by:Gloveboxes Group based inAmesbury, MASSACHUSETTS (USA)
Gloveboxes Group's solutions include tailor-made laminar-flow biosafety cabinets, weighing booths, and transfer trolleys, ideal for applications like nanoparticle cancer drugs and surgical implants. These isolators provide a controlled environment that minimizes cross-contamination and optimize energy use, offering a robust solution for cleanroom and powder ...
Manufactured by:Avery Biomedical Devices, Inc. based inCommack, NEW YORK (USA)
Typically, these patients have high spinal cord injuries, central sleep apnea or other central neurological disorders or a paralyzed diaphragm. A diaphragm pacing system consists of surgically implanted receivers and electrodes and an external transmitter with antennas worn directly over the implanted receivers. The external transmitter and ...