Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Swab Virus Swab Kit Equipment Supplied In Usa
10 equipment items found
Manufactured by:Path-Tec, LLC based inMidland, GEORGIA (US) (USA)
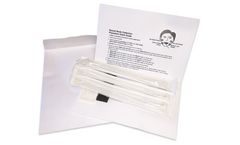
Printed Collection Card, Foam Insert, (3) Buccal Swabs, Printed Envelope, (3) Patient ...
Manufactured by:Creative Diagnostics based inShirley, NEW YORK (USA)
The Strep B Rapid Test is a rapid visual immunoassay for the qualitative presumptive detection of Group B Streptococcal antigen in female vaginal swab. This kit is intended to be used as an aid in the diagnosis of Group B Streptococcal ...
Manufactured by:Alexeter Technologies, LLC based inChicago, ILLINOIS (USA)
Designed with the Emergency First Responder in mind, the Alexeter Collection Swab combines on-site sampling of biological agents and preparation of the sample for field testing in our BioDetect Test Strip. The device includes a Dacron®-tipped swab, self-contained test buffer, pre-filter and transfer sample dropper all contained in one device. The Alexeter Collection Swab System eliminates ...
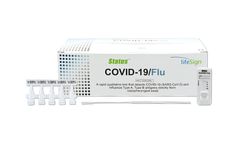
Distributed by:Hardy Diagnostics based inSanta Maria, CALIFORNIA (USA)
A rapid immunoassay for the simultaneous direct detection and differential diagnosis of SARS-CoV-2, Influenza Type A and Type B antigen from anterior nasal (AN) or nasopharyngeal (NP) swab specimens. Kit includes extraction buffer vials and flocked nasopharyngeal (NP) swabs for superior specimen collection and patient comfort, and provides a visual read-out in 15 minutes with no equipment needed! ...
Manufactured by:Rhinostics Inc. based inBoston, MASSACHUSETTS (USA)
Collection device containing innovative and proprietary, full molded flexible sterile nasal swab with break-off tip and capped transport and storage tube. Release your lab from the constraints of the past. Polypropylene nasal swabs provide comparable results to nasopharyngeal collection with additional benefits of sample ...
Manufactured by:PreciGenome LLC based inSan Jose, CALIFORNIA (USA)
A quantitative PCR instrument is a machine that amplifies and detects DNA. It combines the functions of a thermal cycler and a fluorimeter, enabling the process of quantitative PCR. Principal performance dimensions of quantitative PCR instruments are thermal control, fluorimetry and sample throughput. ...
by:GENETWORx, LLC. based inGlen Allen, VIRGINIA (USA)
GENETWORx is your one stop solution for COVID-19 testing. We now offer COVID-19 nasal swab self-collection test kits, in addition to on-site testing and COVID-19 management software. The GENETWORx COVID-19 Nasal Swab Test Kit enables your employees, students, residents and patients to collect a sample at home, send it to the lab, and receive convenient, accurate, and timely COVID-19 diagnostic ...
Manufactured by:Ellume Limited based inEast Brisbane, AUSTRALIA
Ellume’s swab+drop COVID-19 Self-Swab Kit allows for the self-collection of nasal samples based on easy to follow, animated step-by-step online instructions. The swab+drop Self-Swab Kit has an entry on the Australian Register of Therapeutic Goods (ARTG). We endeavor to work with pathology laboratories to make this product available to Australian patients referred for ...
Manufactured by:Gold Standard Diagnostics based inDavis, CALIFORNIA (USA)
The GSD NovaGen SARS-CoV-2 Ag Rapid Test (Nasal Swab) is a single-use test kit intended to detect the presence of the SARS-CoV-2 virus, with self-collected nasal swab specimen from symptomatic individuals who are suspected of being infected with COVID-19. A positive result indicates SARS-CoV-2 antigens present in the ...
Manufactured by:Hologic, Inc. based inMarlborough, MASSACHUSETTS (USA)
The emergence of SARS-CoV-2 was unforeseen, and the worldwide outbreak of COVID-19 has already impacted millions. In response to the desperate public need for rapid high-volume testing we developed SARS-CoV-2 assays on both our Panther® and Panther Fusion® systems.* Both assays enable labs to run over 1000 tests in 24 hours and attain first results in 3.5 hours or ...