Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Test Point Of Care Testing Equipment Supplied In Usa
61 equipment items found
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
The DPP® Micro Reader is a handheld device that enables testing for SARS-CoV-2 and other DPP® tests. Our reader streamlines the point-of-care testing experience with precise and accurate ...
Manufactured by:Biomerica, Inc. based inIrvine, CALIFORNIA (USA)
Available as single Test Strip, single Test Device, or Multi Panel (2-12 on one ...
Manufactured by:Biomerica, Inc. based inIrvine, CALIFORNIA (USA)
Available as single Test Strip, single Test Device, or Multi Panel (2-12 on one ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
Contact activation - Designed for seamless sampling. Unistik® Touch is designed to make capillary sampling as simple and seamless as possible. With one-touch activation, you simply press the device against the sample site, allowing it to activate automatically under pressure. Once activated, the needle retracts into the body of the device, minimizing the risk of re-use, cross-infection and ...
Manufactured by:Biomerica, Inc. based inIrvine, CALIFORNIA (USA)
Available as single Test Strip, single Test Device, or Multi Panel (2-12 on one ...
Manufactured by:Biomerica, Inc. based inIrvine, CALIFORNIA (USA)
Available as single Test Strip, single Test Device, or Multi Panel (2-12 on one ...
Manufactured by:Sekisui Diagnostics, LLC based inBurlington, MASSACHUSETTS (USA)
Detects elevated vaginal fluid sialidase activity, an enzyme produced by bacterial pathogens associated with bacterial vaginosis including Gardnerella, Bacteroides, Prevotella and ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
The DPP® Micro Reader II is our newest, CE Marked, handheld device that enables testing for SARS-CoV-2. Our reader streamlines the point-of-care testing experience with precise and accurate ...
by:Diabetomics, Inc. based inHillsboro, OREGON (USA)
The first and only point-of-care tests for early detection of type-1 diabetes and LADA (Latent Autoimmune Diabetes in ...
by:Diabetomics, Inc. based inHillsboro, OREGON (USA)
The Lumella® Test, The New Point-of-Care Blood Test Helps in Early Detection of Preeclampsia; The Lumella® test estimates the concentration of Glycosylated Fibronectin, a new pregnancy specific biomarker to accurately assess the risk of preeclampsia. The Lumella® test requires a ...
Manufactured by:Sandstone Diagnostics, Inc. based inPleasanton, CALIFORNIA (USA)
Everything you need to collect and process fingerstick capillary plasma or serum with the Torq ZDrive ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
A CE Marked rapid point-of-care test that simultaneously and separately detects treponemal and nontreponemal ...
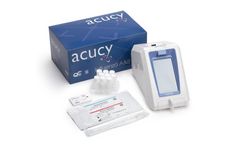
Manufactured by:Sekisui Diagnostics, LLC based inBurlington, MASSACHUSETTS (USA)
With the Acucy® Influenza A&B Test, clinicians can test and provide fast and accurate results that aid in the treatment of a patient in one office visit, enhancing the patient experience, reducing the spread of infection and improving patient ...
by:Diabetomics, Inc. based inHillsboro, OREGON (USA)
First rapid point-of care test for detection of rheumatoid arthritis; Non-invasive oral fluid or finger stick sample; Results in 15 minutes; ACA rapid, point-of-care test for assessment of anti-citrullinated human serum albumin (HSA) autoantibodies (ACAs) and Anti-citrullinated peptides auto ...
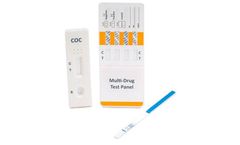
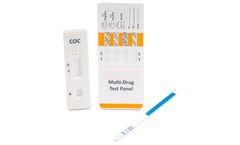
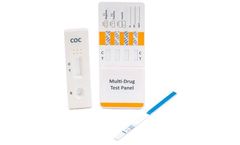
Manufactured by:Alfa Scientific Designs, Inc based inPoway, CALIFORNIA (USA)
ALFA’s reliability for speed and accuracy in point of care rapid tests is readily available in the configuration you need. Our Multi-Drug Urine Cassette Test formats vary to our customers’ ...
Manufactured by:Sandstone Diagnostics, Inc. based inPleasanton, CALIFORNIA (USA)
Avoid hemolysis and ensure lab-quality capillary plasma at the point-of-collection with Sandstone’s Torq MiniDisc and MiniDrive System. The MIniDisc provides clean, liquid plasma from 300uL whole blood in just 2 minutes following ...
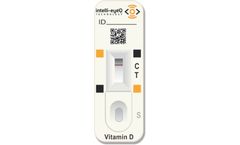
Manufactured by:Spark Mobile Diagnostics based inJamnagar, INDIA
Lateral flow “Sandwich” Assay with technology developed by US experts in industry; Detects both Vitamin D2 and D3 in range of 4 ng/ml – 100 ng/ml; High specificity – No Interference of Biotin; Ideal screening test for Physicians clinics, OB/GYN, Orthopedics and general wellness ...
Manufactured by:HemoSonics, LLC based inDurham, NORTH CAROLINA (USA)
The next generation in Point-of-Care viscoelastic coagulation testing. Rapid results for timely, informed treatment ...
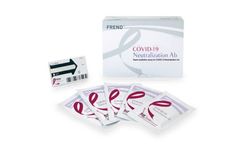
Manufactured by:NanoEnTek, Inc. based inGuro-gu, SOUTH KOREA
The FREND COVID-19 Neutralization Ab is a point-of-care testing (POCT) which can be used to check whether a patient has developed the immune response to SARS-CoV-2 using human serum or plasma ...
Manufactured by:Trukera Medical, formely known as TearLab based inEscondido, CALIFORNIA (USA)
TearLab® Osmolarity System - An Essential Diagnostic for Dry Eye Disease. TearLab is an objective and quantitative point-of-care diagnostic test that provides precise and predictive information. The TearLab Osmolarity System* is intended to measure the osmolarity of human tears to aid in the diagnosis of dry eye disease in patients suspected ...