Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Trauma Product Equipment Supplied In Usa
14 equipment items found
by:FX Shoulder USA based inDallas, TEXAS (USA)
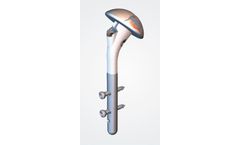
The Humelock II Hemi is a 135° neck shaft angle platform-style convertible stem for trauma cases only. The stem (120mm) has a Unique-To-Market fixation with distal interlocking screws (18mm to 36mm) that allow the surgeon to set the Height & Version intraoperatively with an aiming guide. The plasma sprayed CP Ti (titanium) and HA (hydroxyapatite) coating proximally is designed to enable ...
by:FX Shoulder USA based inDallas, TEXAS (USA)
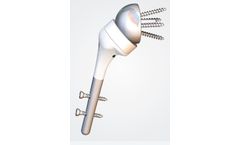
The Humelock Reversed stem (120mm) was originally designed for primary reverse option as well as for trauma application with a 145° neck shaft angle. The innovative design gives optimal fixation proximally and allows for intra-operative options. With its tulip-shape and the plasma sprayed CP Ti (titanium) and HA (hydroxyapatite) coating proximally, it is designed to promote cementless long ...
by:FX Shoulder USA based inDallas, TEXAS (USA)
The PRCT II is a low-profile and simplistic approach to treating proximal humerus fractures. Surgeon-designed from anatomic scans with simple, concise, instrumentation for ease of ...
by:FX Shoulder USA based inDallas, TEXAS (USA)
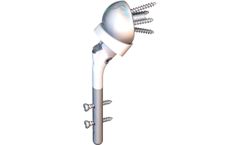
The Humelock II ® Reversible is a convertible stem with a 135° neck shaft angle platform-style convertible stem for trauma cases only. The stem (120mm) has a Unique-To-Market fixation with distal interlocking screws (18mm to 36mm) that allow the surgeon to set the Height & Version intraoperatively with an aiming guide. The plasma sprayed CP Ti (titanium) and HA (hydroxyapatite) ...
Manufactured by:Amplify Surgical based inIrvine, CALIFORNIA (USA)
The dualX systems transform the fusion environment from insertion to spinal restoration by delivery a powerful dual-expanding implant through a minimally-invasive surgical corridor designed to reduce anatomical trauma and nerve retraction. ...
Manufactured by:Amplify Surgical based inIrvine, CALIFORNIA (USA)
The dualX systems transform the fusion environment from insertion to spinal restoration by delivery a powerful dual-expanding implant through a minimally-invasive surgical corridor designed to reduce anatomical trauma and nerve retraction. ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
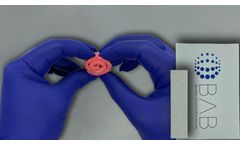
Bi-Ostetic Foam™ is a sterile bone graft composed of highly purified fibrillar Type I bovine collagen and Bi-Ostetic™ resorbable 60% hydroxyapatite and 40% tricalcium phosphate granules. It functions as an osteogenic stimulus to which the patient’s bone marrow can be added, prior to implantation. Bi-Ostetic Foam™ is safe and has excellent biocompatibility. After it is ...
Manufactured by:Medtrica Solutions Ltd based inSurrey, BRITISH COLUMBIA (CANADA)
Hill Rom mattress is designed for maximum comfort and minimal interference to transfer wounded patients in hospital & clinics. We offer a huge range of Hill Rom stretcher mattresses with the mobility and durability of a stretcher. Our Hill Rom transtar electric mattresses are anti-bacterial, anti-fungal, anti-static, stain and tear resistant and made from 4 way stretch ...
Manufactured by:Berkeley Advanced Biomaterials (BAB) based inBerkeley, CALIFORNIA (USA)
Bi-Ostetic Bioactive Glass Foam™ is a sterile bone graft composed of highly purified fibrillar Type I bovine collagen, 45S5 bioactive glass granules and Bi-Ostetic™ resorbable 60% hydroxyapatite and 40% tricalcium phosphate granules. It functions as an osteogenic stimulus to which the patient’s bone marrow can be added to, prior to implantation. Bi-Ostetic Bioactive Glass ...
Manufactured by:Lohmann & Rauscher GmbH & Co. KG (L&R) based inRengsdorf, GERMANY
The Debrisoft Lolly has been developed for the debridement of deep wounds, including those resulting from invasive surgery. This innovative product from L&R is very easy to use and now enables effective debridement, even in hard-to-reach ...
Manufactured by:Xtant Medical based inBelgrade, MONTANA (USA)
The Axle Interspinous Fusion System is an internal fixation device for posterior spinal surgery in the non-cervical spine (T1-S1 inclusive). It is a minimally invasive, modular Interspinous fusion system with straightforward instrumentation. The Axle Interspinous Fusion System is designed to provide spinal stability for lumbar fusion procedures, including the treatment of degenerative disc ...
Manufactured by:Nelipak based inCranston, RHODE ISLAND (USA)
Custom designed ergonomic device trays that save time during surgical procedures and simplify the logistics process. Our design professionals understand the needs of medical device customers and hospital staff for packaging which is easy to use, reduces handling and saves time. End users demand solutions which assure sterility, reduce risk of error during procedure and minimizes ...
by:AVM Biotechnology based inSeattle, WASHINGTON (USA)
Breathing becomes difficult and oxygen cannot get into the body. Most people who get ARDS are already at the hospital for trauma or illness. Lung injuries can occur from trauma, an overwhelming immune response due to an infection, allergic reaction or a cell/gene therapy product or trauma, accidents, ...
Manufactured by:Vascudyne, Inc. based inStillwater, MINNESOTA (USA)
Synthetic vascular grafts currently used in clinic have poor long term outcomes and low patency rates. Hyperplasia, calcification, foreign body immune response, infection, and atheroma formation are commonly encountered limiting the use of synthetic grafts, with none available for small diameter applications like coronary ...