Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Uterine Cavity Equipment Supplied In Usa
11 equipment items found
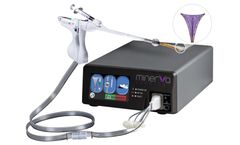
Manufactured by:Minerva Surgical, Inc. based inSanta Clara, CALIFORNIA (USA)
Continuous visualization gives you the ability to see and treat the uterine cavity during the entire procedure. The simple setup and compact design ensures quick case turn around. ...
Manufactured by:Strauss Surgical LLC based inMiami, FLORIDA (USA)
Achieve precise visualization and assessment of the uterine cavity with our 2.9mm diagnostic set. Designed for comfort and clarity, this slender instrument offers gentle access for minimally invasive procedures in outpatient and office ...
Manufactured by:Gyn Surgical Solutions based inMarlborough, MASSACHUSETTS (USA)
MyoSure LITE device: Visualize the entire uterine cavity to get a quality tissue specimen; remove small polyps up to 3 cm with minimal ...
Manufactured by:JUNE Medical USA Inc based inNew Castle, DELAWARE (USA)
Wing Needle For Hysteroscopic Intrauterine Anaesthesia helps to reduce the pain associated with distension and / or manipulation of the uterine cavity during hysteroscopic gynaecology ...
Manufactured by:Femasys Inc. based inSuwanee, GEORGIA (US) (USA)
FemVue is the first FDA-cleared product that creates natural saline and air contrast and enables safe, reliable, and real time evaluation of the fallopian tubes with ultrasound. When performed with a uterine cavity assessment, a more comprehensive exam can be achieved from the comfort of the GYN’s ...
Manufactured by:JUNE Medical USA Inc based inNew Castle, DELAWARE (USA)
Flowable gel that is intended to serve as a temporary, absorbable mechanical barrier separating surgically traumatized opposing tissue surfaces in the uterine cavity where adhesions could potentially ...
Manufactured by:Gyn Surgical Solutions based inMarlborough, MASSACHUSETTS (USA)
It uses controlled radiofrequency energy, heat, to cause coagulative necrosis of the fibroid tissue. The treated tissue softens and shrinks over time, allowing fibroid symptoms to resolve with no suturing of the uterine tissue. Unlike many alternative interventions, the Acessa procedure optimizes imaging of the uterus by simultaneously displaying the laparoscopic camera view and ...
Manufactured by:AEGEA Medical Inc. based inMenlo Park, CALIFORNIA (USA)
The Mara Water Vapor Ablation System is redefining endometrial ablation. By gently filling the uterine cavity with naturally expansive water vapor, Mara treats more women with varying uterine anatomies*, does not require general anesthesia, and most importantly, safely and effectively reduces heavy menstrual bleeding. *Cavity ...
Manufactured by:Minerva Surgical, Inc. based inSanta Clara, CALIFORNIA (USA)
Experience a modern device that delivers complete and consistent depth of coverage, without using RF energy only to ablate endometrial ...
Manufactured by:LiNA Medical ApS. based inGlostrup, DENMARK
UNA Librata™ Endometrial Ablation Simplified; -2 minute thermal treatment time, Slim 5.4 mm catheter requires minimal or no dilation, Ideally suited for ambulatory gynecology, Only a simple sounding measurement ...
Manufactured by:Femasys Inc. based inSuwanee, GEORGIA (US) (USA)
FemBloc features our proprietary delivery platform, which places balloon technology close to the opening of both fallopian tubes. This in-office approach is designed to eliminate the risks of incisions, anesthesia, and hormones. As a nonsurgical procedure that is implant free, FemBloc delivers a biopolymer that is expected to expel within 3 months. Confirmation of procedure success can be ...