Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Vaccine Development Equipment Supplied In Usa
48 equipment items found
Manufactured by:JPT Peptide Technologies GmbH based inBerlin, GERMANY
Neo-epitopes are important targets for individualized cancer immunotherapy. Recent advancements in next generation sequencing and bioinformatic approaches to predict immunogenicity of neo-epitopes improved target selection for therapy. However, in many cases only a fraction of predicted epitopes generate a specific T-cell response. Therefore, various assay techniques such as ELISpot, ...
Manufactured by:Novavax based inGaithersburg, MARYLAND (USA)
Novavax' saponin-based Matrix-M improves immune responses and enables vaccine dose-sparing. Matrix-M adjuvant is a valuable component of vaccine development, providing multiple immune system enhancements, a well-understood mechanism of action, and robust clinical experience where it was shown to be well tolerated in human studies to ...
Manufactured by:POP Biotechnologies, Inc. (POP BIO) based inBuffalo, NEW YORK (USA)
The SNAP (Spontaneous Nanoliposome Antigen Particleization) platform technology is a unique liposome-based system that functions as a potent vaccine adjuvant designed to address functional and practical limitations facing vaccine ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has developed a VesiculoVax™-vectored vaccine to protect against the arthralgic disease caused by infection with Chikungunya ...
by:BacVax, Inc. based inPhoenix, ARIZONA (USA)
Current commercial bacterial vaccines use toxin neutralization, capsule targeting, or multiple proteins. While these methods may work in some circumstances, many bacteria do not have toxin or capsule targets, and using several proteins in a vaccine is expensive and opens the opportunity for genetic escape by the bacteria. ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines is developing a GeneVax prime/VesiculoVax boost therapeutic vaccine for herpes simplex virus type-2. This vaccine is designed to ameliorate disease and transmission by preventing HSV-2 reactivation and ...
by:Revvity Gene Delivery (formerly SIRION Biotech) based inHamburg, GERMANY
Adenoviral vectors are the method of choice whenever fast and transient gene expression for a very large gene or several genes is required, since the expression cassette has a total capacity of 7.5 kb. This provides space for flexible vector design with multiple transgenes delivered in one vector. Vaccine development is another popular application for adenoviral ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has developed a VesiculoVax™-vectored vaccine to protect against Lassa hemorrhagic ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
As part of its comprehensive Public Health and Biodefense vaccine franchise, under a license from the Henry M. Jackson Foundation, Auro Vaccines is developing a subunit vaccine (HeV-sG-V) composed of the envelope glycoprotein of Hendra virus for the prevention of Nipah virus (NiV) ...
Manufactured by:Ardigen based inKraków, POLAND
Quick solution for effective immune responses in clinical trials; We have created an Artificial Intelligence platform dedicated to being a part of the therapeutic cancer vaccine development. The ArdImmune Vax platform uses sequencing data to generate and prioritize antigen targets for cancer vaccines and T-cell ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines is developing a GeneVav prime/VesiculoVax™ boost therapeutic vaccine that targets all HCV genotypes for use as an adjunct to drug ...
Manufactured by:JPT Peptide Technologies GmbH based inBerlin, GERMANY
Clinical peptides and PepMix Peptide Pools are increasingly needed as antigen source for clinical immune monitoring, diagnostics, cell therapy and vaccine development. The diversity of applications and regulatory requirements calls for a flexible, high quality peptide partner. JPT is that partner with its outstanding know-how on peptide antigen formats, peptide ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines is developing a GeneVax prime/VesiculoVax boost therapeutic HPV vaccine for use as an adjunct to surgery that targets the seven HPV types responsible for more than 92% of cervical and head and neck ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has developed VesiculoVax™-vectored vaccines for pre- and post-exposure protection against the hemorrhagic disease caused by multiple species of filovirus (i.e. Zaire ebolavirus, Sudan ebolavirus and Marburg ...
Manufactured by:Elicio Therapeutics based inBoston, MASSACHUSETTS (USA)
ELI-008 is a multivalent lymph node–targeted AMP peptide vaccine developed to target p53 hotspot mutations. p53 is a tumor-suppressing protein that controls the cell cycle, DNA replication, and uncontrolled cell division during tumor growth.4 Mutations in the p53 protein lead to uncontrolled tumor progression and growth.4 Similar to Kristen ...
Manufactured by:United Biomedical based inNew York, NEW YORK (USA)
Development of vaccines for human health based on a synthetic peptide platform, spanning therapeutic areas such as neurology, allergy, and infectious ...
by:Merck & Co., Inc., based inRahway, NEW JERSEY (USA)
BCG VACCINE for percutaneous use is an attenuated, live culture preparation of the Bacillus of Calmette and Guerin (BCG) strain of Mycobacterium bov/s.{1} The TICE® strain used in this BCG VACCINE preparation was developed at the University of Illinois from a strain originated at the Pasteur ...
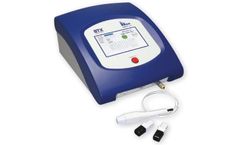
Manufactured by:BTX - Harvard Bioscience, Inc. based inHolliston, MASSACHUSETTS (USA)
The AgilePulse In Vivo System, used for animal immunization, vaccine development, and gene therapy, provides an intra-muscular, intra-dermal, or intra-tumor electroporation solution to produce maximum transfection efficiency. Traditional in vivo nucleic acid delivery systems, such as gene gun delivery, suffer from poor efficiency. ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has also developed a GeneVax prime/VesiculoVax boost therapeutic vaccine that targets all eight HBV genotypes for use as an adjunct to drug ...
Manufactured by:Elicio Therapeutics based inBoston, MASSACHUSETTS (USA)
ELI-007 is a multivalent lymph node–targeted AMP peptide vaccine developed to target BRAF gene mutations. The BRAF gene is part of an intracellular signaling pathway that drives cell growth and division.1 BRAF mutations can lead to uncontrolled cell growth and ultimately cancer. BRAF mutations are present in multiple types of cancer, including 40% ...