Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Vein Dilation Equipment Supplied In Usa
3 equipment items found
Manufactured by:Artio Medical, Inc. based inPrairie Village, KANSAS (USA)
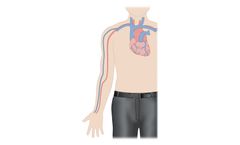
Innovative vein dilation system for hemodialysis patients. The Amplifi Vein Dilation System is designed to stimulate arm vein enlargement in hemodialysis patients using rapid, non-pulsatile blood flow. The Amplifi System aims to increase eligibility for arteriovenous fistula (AVF) surgery, reduce AVF maturation ...
Manufactured by:Boehringer Ingelheim Animal Health USA Inc. based inDuluth, GEORGIA (US) (USA)
VETMEDIN (pimobendan) Chewable Tablets and VETMEDIN Solution (pimobendan oral solution) are indicated for the management of the signs of mild, moderate, or severe congestive heart failure (CHF) in dogs due to clinical myxomatous mitral valve disease (MMVD) or dilated cardiomyopathy (DCM). Both formulations of VETMEDIN are indicated for use with concurrent therapy for congestive heart failure ...
Manufactured by:Boehringer Ingelheim Animal Health USA Inc. based inDuluth, GEORGIA (US) (USA)
VETMEDIN-CA1 (pimobendan) Chewable Tablets are indicated for the delay of onset of congestive heart failure in dogs with Stage B2 preclinical myxomatous mitral valve disease (2019 American College of Veterinary Internal Medicine Consensus Statement). It is a violation of Federal law to use VETMEDIN-CA1 other than as directed in the labeling. Conditionally approved by FDA pending a full ...