

Forge Biologics, Inc.
- Home
- Companies
- Forge Biologics, Inc.
- Services
- AAV Manufacturing Services
AAV Manufacturing Services
We offer a robust platform approach wholly built for AAV production. That means you have access to a leadership team with 165+ years of industry experience, 200,000+ sq. ft. cGMP facility, proprietary technologies optimized for safety, and a company that shares our clients’ missions of advancing potential treatments for those who need them the most.
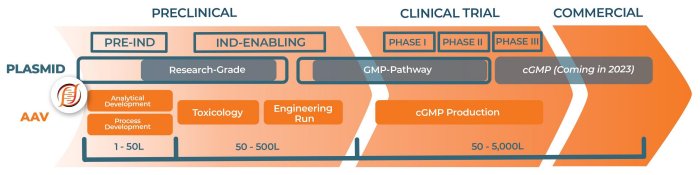
Blaze Vector Production is our turnkey, scalable, research-grade AAV production
- Our proprietary Ignition Cells™ have a clear, well-documented history and are optimized for scalable suspension growth
- Established process for many standard AAV serotypes
- Upfront communication and pricing
- Fast turnaround time
End-to-end capabilities from research to cGMP reduce the need for bridging studies
- Our experienced team supports process development and scale up
- In-house analytical assay development and performance to support cGMP-readiness
- Process development to manufacture novel engineered capsids
- View our analytical development assays
Fully disposable, closed system processing
- Flexible scheduling and maximization of capacity
- Consistent process and product flow for reduction of variation
- 50-5,000L scale. See our 5,000L bioreactor.
