- Home
- Services
- north korea
- toxicology
Show results for
Refine by
Toxicology Services Available In North Korea
20 services found
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
A successful ADC should be chemically and physiologically stable in blood stream, exert similar antigen binding pattern compared with the unconjugated antibody, and exhibit desired payload toxicological effects once internalized. ...
Manufactured by:Puracyp based inCarlsbad, CALIFORNIA (USA)
Puracyp’s cells contain the promoter/enhancer of these three P450 enzymes in addition to either CAR, AhR, or PXR. Panel screens are also useful for examining species differences in P450 induction and for toxicology studies using orthologs of human nuclear receptors. An example of results obtained in human cell lines utilizing our panel screen for PXR, AhR and CAR are shown ...
by:PharmaLex GMBH based inFriedrichsdorf, GERMANY
Going far beyond basic project management, the Integrated Product Development program at PharmaLex represents a partnership between you and our team. Our experts actively contribute to your product’s path to success by integrating nonclinical/clinical, toxicological and crucial biostatistical support with Chemistry, Manufacturing and Controls (CMC) services, regulatory ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
Zebrafish have become the third largest vertebrate model organism after mouse and rat, and have been widely used in many fields such as developmental biology, genetics, basic medicine, pharmacology, toxicology, drug research and development, and ecological environment evaluation. Creative Biolabs provides a one-stop service platform for zebrafish gene knockout/knock-in and ...
Manufactured by:Novodiax, Inc. based inHayward, CALIFORNIA (USA)
Use the Novodiax polymerized horseradish peroxidase (polyHRP) signal amplification technology (ihcDirect) and conjugate it directly to your humanized antibody-of-choice to enable rapid, sensitive IHC testing. Jumpstart your early humanized antibody-to-human tissue testing or ADME/toxicology studies to fail candidates ...
Manufactured by:ExcellGene SA based inMonthey, SWITZERLAND
Have you been limited a lack of tox material to properly design your pre-clinical toxicology studies in animals? We will help you plan experiments without the worry of running short. We can deliver up to 500 grams of drug substance from a single batch, in 8 months or less. Our scientific foundation is built on expert knowledge, dependable quality, and timely delivery. ...
Manufactured by:Werfen based inL`Hospitalet de Llobregat, SPAIN
We offer easy-to-use clinical laboratory instrumentation and reagents for chemistry and pharma-toxicology labs. Our unique solutions are used to diagnose conditions such as diabetes as well as to identify drug ...
by:Andelyn Biosciences based inColumbus, OHIO (USA)
Andelyn Biosciences has more than 20 years of experience in cell and gene therapy manufacturing. We possess the capability and capacity to manufacture batches of plasmids in any size depending on client specifications – from small trials to clinical-scale plasmid ...
by:Emmace Consulting AB based inLund, SWEDEN
Testing and determination of Particulate Matter (PM) and Volatile Organic Substances (VOS) according to ISO 18562-2 and ISO ...
Manufactured by:Chanelle Pharma Group based inCo Galway,, IRELAND
At Chanelle, our in-house research and development capabilities are the cornerstone of our operations and continued growth. While we are a generic medicines provider, our commitment to innovation is comparable to brand leaders in the ...
Manufactured by:Germaine Laboratories, Inc. based inSan Antonio, TEXAS (USA)
Our Drugs of Abuse Testing division has many years’ experience assisting major employers, School Districts, Criminal Justice and Drug Court entities throughout the United States. Our laboratory staff is dedicated to providing you with reliable results when you need ...
by:Emmace Consulting AB based inLund, SWEDEN
ISO 18562-4 addresses the critical need for quantifying and assessing the risks associated with condensate produced during patient use, which can potentially enter the lungs or the throat region depending on the device. Accurate measurement of the condensate volume is essential for ensuring patient safety, meeting regulatory requirements and making it possible to estimate the exposure to ...
by:Selvita S.A. based inKrakow, POLAND
Having experience in both pharma and biotech R&D, we are well aware of what it means to discover a hit and transform it into a drug. You can best benefit from this “insider” experience through our integrated drug discovery services built ...
by:Andelyn Biosciences based inColumbus, OHIO (USA)
At Andelyn Biosciences, we’re an ideal viral vector CDMO partner. We work closely with you throughout the entire process of developing your cell and gene therapy or vaccine – from the initial process of development and research to creating a reliable and consistent clinical and commercial supply. Andelyn Biosciences is with you every step of the ...
Manufactured by:InnoSer Belgie NV based inDiepenbeek, BELGIUM
In vitro and in vivo methods to provide a study setup that thoroughly determines safety and efficacy. ...
by:Andelyn Biosciences based inColumbus, OHIO (USA)
Andelyn Biosciences provides clients with a broad range of viral vector manufacturing platforms and solutions, including AAV and Lentiviral adherent and suspension systems along with plasmid manufacturing. We support our clients throughout every step of cGMP manufacturing – from pre-clinical trials of gene-modified products and cell therapies to commercial ...
Manufactured by:InnoSer Belgie NV based inDiepenbeek, BELGIUM
We optimize your lead compounds using expertise and data-driven decisions. The lead optimization stage marks the end of the drug discovery phase and the start of pharmaceutical or biological development. Insight into the absorption, distribution, metabolism, and excretion (ADME) is key to progressing your identified lead compounds after the hit to lead phase. ...
Manufactured by:InnoSer Belgie NV based inDiepenbeek, BELGIUM
We move your identified hits forward into in vivo testing and lead optimization. As a critical phase in your drug development journey, the hit to lead phase will determine which of your promising compounds will be characterized against your targets. With a lean ethos, InnoSer is dedicated to combining our expertise with your drug development goals in the most resource efficient way. During this ...
Manufactured by:LAMPIRE Biological Laboratories, Inc. based inPipersville, PENNSYLVANIA (USA)
LAMPIRE's high-tech vivarium and staff are available for your lab animal projects. The expanded capacity and enhanced facilities can accommodate drug metabolism and pharmacokinetic (DMPK) studies, teratogenicity studies, small- and large-scale hybridoma projects, and much more. One of the biggest challenges in preclinical research is procuring high quality animal services to support your drug ...
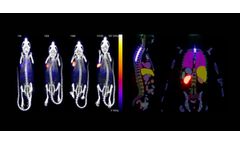
Manufactured by:Invicro, LLC based inNeedham, MASSACHUSETTS (USA)
Radiopharmaceutical therapies represent an effective way to treat solid cancers by using tumor targeting small molecules, peptides or biologics to deliver a cytotoxic payload that induces DNA damage in tumor cells, while limiting damage to normal and healthy tissue. Streamlined development, evaluation and clinical translation of radiopharmaceuticals requires a partner that has extensive ...