- Home
- Services
- north america
- administration of drug
Refine by
Administration Of Drug Services In North America
43 services found
Manufactured by:SCIREQ - an emka TECHNOLOGIES Company based inMontreal, QUEBEC (CANADA)
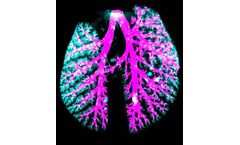
VAAD is a new innovative technique using the flexiVent, introduced to improve in vivo aerosol administrations for drug delivery and disease modelling. The VAAD approach minimizes subject-to-subject variability, and fosters a highly homogenous aerosol deposition within the lung. This optimized aerosol protocol is synced with mechanical ventilation to standardize ...
by:German American Chamber of Commerce, Inc. based inNew York, NEW YORK (USA)
German companies planning a US Market Entry are often confronted with numerous legal, tax and economic challenges. One of the most common reasons for a US Market Entry to fail is often not the business idea or the product itself but a lack of information and diligence when it comes to implementation. A successful US Market Entry requires therefore special planning to avoid the possible ...
Manufactured by:Laxxon Medical based inNew York, NEW YORK (USA)
Through our proprietary SPID®-Technology, we can repurpose, reposition, and relaunch generic APIs with patent protection while also addressing common issues with generic drugs such as patient compliance, dosage, and side effects. Our Advanced Patented Generics are going to be registered in the US under the FDA Section 505(b)(2), a fast-tracked regulatory approval process. In the EU, our ...
Manufactured by:Integer Holdings Corporation based inPlano, TENNESSEE (USA)
Our commitment to quality ensures that our products and services meet or exceed the highest standards set by the United States Food and Drug Administration (FDA), the European Community, and other internationally recognized agencies. We maintain an industry-wide reputation as an award-winning manufacturer with high quality standards through enforcement of ...
by:HDR Inc. based inOmaha, NEBRASKA (USA)
Our architects, engineers, designers and planners are inspired by our clients and their work. We are honored to design facilities where scientists may someday discover a cure for cancer, where caregivers provide a healing environment for injured U.S. soldiers returning from deployment, and where the fundamental acts of the United States Judicial System are demonstrated in real life each ...
by:Cyrus Biotechnology Inc. based inSeattle, WASHINGTON (USA)
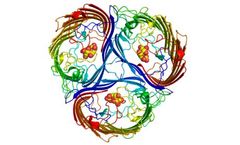
Protein design has evolved tremendously over the last 20 years and Rosetta has been at the bleeding edge throughout that time. Rosetta was first at most major protein design achievements, such as protein stabilization, affinity, enzyme specificity, novel protein assemblies, and even entirely new protein structures with no sequence similarity to natural ...
by:Igenbio, Inc. based inChicago, ILLINOIS (USA)
Igenbio has provided support for both European Food Safety Authority (EFSA) and Food and Drug Administration (FDA) regulatory filing of Generally Recognized as Safe (GRAS) ...
by:Omnimed based inCookshire-Eaton, QUEBEC (CANADA)
This research project seeks the participation of general practitioners wishing to contribute to the advancement of research on the treatment of anemia in women aged 18 to 45 years. This project is led by the research team of the research institute of the McGill University Health ...
by:Kalium Solutions based inBoucherville, QUEBEC (CANADA)
We can obtain for you a Natural Product Number (NPN) as well as a site licensing (SL) by Health Canada for your ethyl or isopropyl alcohol-based hand sanitizers. We can also get its US equivalent, a registration number (NDC registration number) from the Food and Drug Administration ...
by:German American Chamber of Commerce, Inc. based inNew York, NEW YORK (USA)
The German American Chamber of Commerce, Inc. in New York acts as U.S. Agent for German companies towards the U.S. Food and Drug Administration ...
Manufactured by:Atrion Corporation based inAllen, TEXAS (USA)
Atrion provides contract manufacturing services for other major original equipment manufacturers of medical devices. Our facilities are registered with the U. S. Food and Drug Administration. Our comprehensive services, including patent assistance and regulatory support, help our customers take their products from idea to ...
by:Asbestos.com, The Asbestos & Mesothelioma Center based inOrlando, FLORIDA (USA)
It took nearly a century of research for doctors to learn what causes this cancer, who is most at risk for contracting it, what symptoms indicate its presence and what tools are most effective at diagnosing it. These strides significantly impacted how medical professionals treat asbestos cancer, but the investigation is hardly finished. Current efforts on developing more effective treatments and ...
Manufactured by:AGC Biologics based inBothell, WASHINGTON (USA)
AGC Biologics has a strong regulatory track record. We have been successfully inspected by the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), the Danish Medicines Agency (DMA), the Paul Ehrlich Institute (PEI), Health Canada and the Pharmaceuticals and Medical Devices Agency (PMDA). The many audits and inspections conducted at AGC ...
Manufactured by:Actylis based inPort Washington, NEW YORK (USA)
We develop sourcing solutions aiming not only at securing your access to strategic ingredients, but also to ensure the ingredients are produced sustainably with the highest guarantee of purity and traceability. For this we rely on a 70 years track record of supply chain integrity and an effective presence in many countries. Our global presence, R&D centers of excellence, and our strategically ...
Manufactured by:Emmaus Medical, Inc based inTorrance, CALIFORNIA (USA)
Under the terms of the agreement, Emmaus Life Sciences and Cellseed will conduct clinical trials to seek Food and Drug Administration approval for use by patients in the United States. ...
by:Igenbio, Inc. based inChicago, ILLINOIS (USA)
Igenbio has provided support for both European Food Safety Authority (EFSA) and Food and Drug Administration (FDA) regulatory filing of Generally Recognized as Safe (GRAS) organisms, including: Whole Genome Sequencing using both long and short read platforms available such as Illumina, PacBio, and Oxford Nanopore. Genome Assembly to EFSA and FDA completion ...
Manufactured by:MorphoSys AG based inPlanegg, GERMANY
At MorphoSys, our mission is to make innovative biopharmaceuticals to improve the lives of patients suffering from serious diseases. Before these and all other investigational medicinal products can be made broadly available, the U.S. Food and Drug Administration (FDA) and other health authorities around the world require that they are investigated in clinical ...
by:Patheon Inc. based inDurham, NORTH CAROLINA (USA)
Broad experience and access to the latest technological capabilities are essential for today’s increasingly complex API development and manufacturing. By partnering with Thermo Fisher Scientific, you’ll work directly with scientists with deep chemistry knowledge and many years of experience in successfully supporting early and later phase clinical development programs. Our experts ...
by:CSA Group based inToronto, ONTARIO (CANADA)
Mitigate the risk of a cyber attack with thorough review and testing of your connected products and systems. Cybercrime damage costs are expected to hit $6 trillion annually by 2021, up from $3 trillion in 2015. IoT devices connect twice as many people that are alive in the world. In 2015, the FBI warned about the dangers of IoT, yet most devices are still considered vulnerable to attack. The ...
Manufactured by:Diamond Diagnostics Inc. based inHolliston, MASSACHUSETTS (USA)
Due to tighter healthcare budgets and increased environmental awareness, many laboratories and distributors are pursuing options to extend the use life of instrumentation they currently own. If you are satisfied with the performance of your analyzer, but would like to enhance its appearance and extend its use life considerably, please consider recycling your analyzer with Diamond Diagnostics. In ...