Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Drug Conjugation Services In Europe
14 services found
by:Revvity Gene Delivery (formerly SIRION Biotech) based inHamburg, GERMANY
In the discovery of oncology therapeutics, Antibody-drug conjugates (ADCs) are rapidly gaining momentum, with hundreds of candidates advancing through clinical development. Whether your priority lies in assessing the epitope binding of your construct, evaluating the cytotoxic potency of the payload, or scrutinizing the efficacy of the linker in cleaving, each ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
Antibody-drug conjugates employ the specific monoclonal antibodies (mAbs) to achieve targeted delivery of the conjugated cytotoxic molecules to tumor cells. This delicate design increases the payload efficacy against tumor cells and in the meantime, reduces the cytotoxicity of the otherwise highly toxic payload to normal tissues comparing to ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
Antibody drugs which consist of antibody substances are prepared by antibody engineering technology based on cell and gene engineering technology. An important feature of antibody drugs is that they can be tailored making by genetic engineering and be prepared according to specific therapeutic needs. With the development of antibody genetic engineering ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
Pharmacokinetics (PK) studies provide critical information regarding the behavior of a drug in circulation and its ultimate form after extensive in vivo metabolism. Results from PK studies often serve as guidelines for clinical trials designs, especially in the development of an antibody-drug conjugate (ADC). Due to the heterogeneous nature of ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
Monoclonal antibody-based immunotherapies against cancer and other infectious diseases are highly advantageous comparing to conventional therapeutic approaches due to their high specificity and affinity towards well-defined targets. Antibody-drug conjugates (ADCs) inherit such superiorities and more remarkably, expand the therapeutic window of the ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
Pharmacokinetics (PK) studies provide critical information regarding the behavior of a drug in circulation and its ultimate form after extensive in vivo metabolism. Results from PK studies often serve as guidelines for clinical trials designs, especially in the development of an antibody-drug conjugate (ADC). Due to the heterogeneous nature of ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
The construction of an antibody-drug conjugate (ADC) is the covalent coupling of a monoclonal antibody and a toxic payload drug via a linker molecule. The ultimate goal for an ADC development project is to deploy the resulted ADC into in vivo systems, both in model animals and eventually human patients, to cure cancer and other diseases utilizing ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
As one major component of an antibody-drug conjugate (ADC), the antibody is the key for target specificity and serves as the cargo to deliver the cytotoxic drug (payload). A payload drug can be attached to different sites on an antibody using diverse conjugation chemistry. Multiple endogenous amino acids can ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
As one major component of an antibody-drug conjugate (ADC), the antibody is the key for target specificity and serves as the cargo to deliver the cytotoxic drug (payload). A payload drug can be attached to different sites on an antibody using diverse conjugation chemistry. Multiple endogenous amino acids can ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
As one major component of an antibody-drug conjugate (ADC), the antibody is the key for target specificity and serves as the cargo to deliver the cytotoxic drug (payload). A payload drug can be attached to different sites on an antibody using diverse conjugation chemistry. Multiple endogenous amino acids can ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
The construction of an antibody-drug conjugate (ADC) is the covalent coupling of a monoclonal antibody and a toxic payload drug via a linker molecule. The ultimate goal for an ADC development project is to deploy the resulted ADC into in vivo systems, both in model animals and eventually human patients, to cure cancer and other diseases utilizing ...
Manufactured by:BOC Sciences based inShirley, NEW YORK (USA)
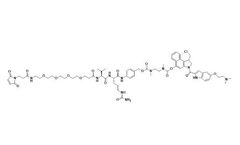
MA-PEG4-vc-PAB-DMEA-duocarmycin DM is a drug-linker conjugate for ADC by using Duocarmycin DM (a potent antitumor antibiotic), linked via MA-PEG4-vc-PAB-DMEA. Resource ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
Creative Biolabs offers engineered protein scaffold library construction service. Our service has provided a strong foundation for the successful isolation and exploitation of high-affinity binders in the fields of research, diagnosis, and therapy. Creative Biolabs has developed trimer codon technology, which offers site-directed mutant library construction in a high-throughput and extremely ...
Manufactured by:Creative Biolabs based inShirley, NEW YORK (USA)
Antibody characterization is important in research and biopharmaceutical development. All therapeutic proteins produced from current technologies have heterogeneities in the final product because various modifications occur during different stages of production. Due to these heterogeneities, thorough characterization is necessary for the reproducible and safe production of therapeutic ...