

CRScube Inc.
cubeCTMS - Clinical Trial Management System for Real-Time Central Monitoring
FromCRScube Inc.
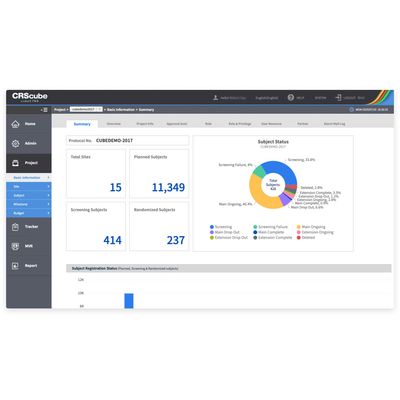
cubeCTMS® is a clinical trial management system for real-time central monitoring allowing users to view and manage information on the progress of a clinical trial and milestone at a glance. Additionally, cubeCTMS® provides users with effective resource allocation and risk-based monitoring can be planned, taking into account risk areas identified as issue tracking. Conduct clinical trials with cubeCTMS®.
Most popular related searches
BENEFITS
- Free basic training
- Notification of issues that may reduce data quality
- Improves workflow, team communication, target enrollment, resources
- Track and view the status of milestones
- Seamless synchronization with cubeCDMS
- Unlimited downloads of over 10 standardized reports at no additional cost
Central monitoring
- Intuitive dashboards
- Schedule and task management with calendar and TO-DO lists
- User-friendly site and subject management
- Issue tracking (SAE, PD/PV)
- Budget tracking
Document Management
- Study documents management: Protocol, ICF, CRF, IRB
- Inquiry and tracking of study document submission status
- MVR management and customization according to study timelines
- Compliance and QA with CRA signature workflows
- MVR Template Customization
- Download MVR Dataset
