Refine by
Clinical Regulations Software
16 software items found
by:Clinion based inHyderabad, INDIA
Using AI to Automate Study & Submission Documentation. Automate Protocol And Clinical Study Reports With The Help Of Machine ...
by:SK-Telemed GmbH based inVienna, AUSTRIA
Creation of EHR- information about the patient from the appointment to the discharge from the hospital is preserved and available to all participants in the treatment process Possibility of integrating EHR with other information systems and registries for obtaining additional information about the patient Intergated- classifiers of medicines, medical services and clinical protocols The ...
by:Etiometry Inc. based inBoston, MASSACHUSETTS (USA)
Etiometry’s Clinical Management Applications (MAPs) are designed to aid clinical staff in all types care settings to deploy clinical protocols and follow patient-care guidelines. Clinical MAPs embed your hospital-established protocols into the T3 Software, while seamlessly integrating with your current workflows. With the protocols readily available for clinicians, increased ...
by:AWS Truepower, LLC based inAlbany, NEW YORK (USA)
Discover how our validated, FDA CFR Part 11 compliance training management system helps you comply with evolving FDA Regulations. Keep your workforce up to date. Medical device and pharmaceutical manufacturers trying to keep up with ever-changing regulations have a tough job. Medical technology advances quickly, and with it the need for compliance systems to ...
by:VIDA Diagnostics Inc. based inCoralville, IOWA (USA)
VIDA provides quality, quantitative CT imaging analysis for all major lung and respiratory diseases to streamline clinical trials with leading ...
by:Jeeva Informatics Solutions Inc. based inManassas, VIRGINIA (USA)
Clinical research sites, sponsors, and CROs, minimize the logistical burdens of paper based process for gathering patient reported outcomes, patient diaries, patient satisfaction, and quality of life questionnaires. Whether standardized or custom on a per-clinical-trial subscription or enterprise license. The modular subscription service is based on number of patients and can scale as the study ...
by:Jeeva Informatics Solutions Inc. based inManassas, VIRGINIA (USA)
Clinical research sites, sponsors, and CROs, minimize the logistical burdens of the informed consent process on a per-clinical-trial subscription or enterprise license. The modular subscription service is based on number of patients and can scale with the study enrollment process. Automate the manual repetitive tasks of explaining the study protocol, risks, benefits, samples or data use, and ...
by:Medidata based inNew York, NEW YORK (USA)
Medidata AI Integrated Evidence connects historical data from more than 23,000 cross-sponsor clinical trials and 7 million patient volunteers with real world data to deliver powerful insights and the necessary evidence clinical development leaders need to increase the probability of trial success. Artificial intelligence and data can help fuel new ways of working on clinical trials and beyond. ...
by:CureMetrix, Inc. based inSan Diego, CALIFORNIA (USA)
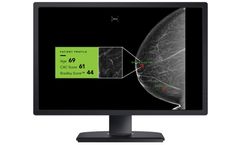
cmTriage™ from CureMetrix is the first FDA-cleared software in the U.S. intended to provide a notification triage code to the radiologist’s mammography worklist based on the presence of a suspicious region of interest found by the underlying algorithm. This workflow optimization tool enables a radiologist to customize their 2D mammogram or mammography worklist based on cases that may ...
by:MVision AI based inHelsinki, FINLAND
Utilizing models adhering to international best practices, Contour+ assures standardized contours which enhance inter-clinic collaboration. It significantly decreases manual effort and contour variability, offering up to a 95% reduction in workload. The software integrates seamlessly with existing treatment planning systems and caters to stringent data protection standards such ...
by:CureMetrix, Inc. based inSan Diego, CALIFORNIA (USA)
cmAngio An investigational AI-based triage software that helps doctors assess a patient’s risk of coronary heart disease ...
by:PathAI, Inc. based inBoston, MASSACHUSETTS (USA)
AISightTM is a web-based digital pathology slide viewing platform that enables the management and analysis of whole slide ...
by:Oasis Technologies Group, LLC. based inLouisville, KENTUCKY (USA)
Are you tired of submitting your billing claims and getting denials? Is it time consuming and distracting you from day to day operations of your agency. At Oasis Technologies, we have teamed up with some of the most respected revenue cycle management companies in the industry to provide you with choices and billing options. We want to help you reduce the cost and stress of billing so you can ...
by:Oasis Technologies Group, LLC. based inLouisville, KENTUCKY (USA)
Effective and meaningful documentation of our services is a key to successful reimbursement and ensuring the delivery of competent care. At Oasis, we provide a general platform for notes, but realize that different types of providers have different needs. An SCL Medicaid provider may have different needs than an outpatient occupational ...
by:Oasis Technologies Group, LLC. based inLouisville, KENTUCKY (USA)
Maintaining accurate and reliable electronic health records is one of the primary goals for Oasis Technologies Group. It can be a daunting and burdensome task maintaining and managing client records. When insurance companies audit records you may become stressed out and overwhelmed. Many times the paperwork and record maintenance responsibilities just gets in the way of providing effective and ...
by:Oasis Technologies Group, LLC. based inLouisville, KENTUCKY (USA)
Staff members and personnel are required to have trainings, certifications and continuing education. In the busy world of providing services some professionals benefit from reminders and a system to maintain current. The Oasis system provides you with solutions to maintain staff training records and provides you with notification for when staff trainings are due. The Oasis system strives to help ...