Show results for
Refine by
Hazardous Situation Software
4 software items found
by:AQA Company, Inc. based inLa Canada, CALIFORNIA (USA)
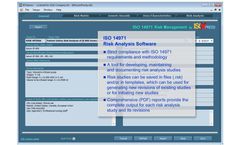
IMSXpress 14971 Medical Device Risk Management software is a Windows application for implementing Risk Analysis, Risk Evaluation, and Risk Control in strict compliance with the ISO 14971:2012 standard. Risk analysis, risk evaluation, and risk control methodologies strictly follow requirements of ISO 14971 and all recommendations included in ISO 14971 Annex B through E. Reports generated by ...
by:Orcanos Israel based inGivatayim, ISRAEL
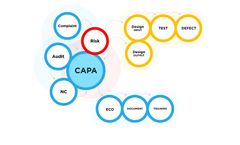
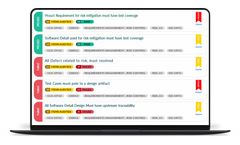
The Orcanos Risk Management tool essentially connects two different modules within the compliance process: Design Control (ALM) and Quality Management. The key to effective Risk Management is to reduce the level of risk that can impact an organization. More often that not, this relies heavily on knowing when a potential problem is about to happen. Orcanos’ Risk Management system has an ...
by:Orcanos Israel based inGivatayim, ISRAEL
Our medical requirements management tool is perfect for manufacturers of medical devices and will provide you with a number of essential quality-related ...
by:Orcanos Israel based inGivatayim, ISRAEL
Adhere to strict MDR/FDA regulations and ISO quality standards by eliminating paper-based QMS ...