Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Drug Enforcement Administration Software In Europe
6 software items found
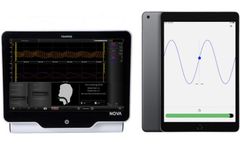
Manufactured by:Finapres Medical Systems based inEnschede, NETHERLANDS
New GAT application enables standardization and quantification! In autonomic testing, standardized procedures are essential for reliable test results. Finapres Medical Systems developed a Guided Autonomic Testing (GAT) application as part of the Finapres® NOVA, which guides the operator and the patient through a series of autonomic test maneuvers. The GAT application consists of a graphical ...
Manufactured by:Finapres Medical Systems based inEnschede, NETHERLANDS
New GAT application enables standardization and quantification! In autonomic testing, standardized procedures are essential for reliable test results. Finapres Medical Systems developed a Guided Autonomic Testing (GAT) application as part of the Finapres® NOVA, which guides the operator and the patient through a series of autonomic test ...
by:MasterControl, Inc based inSalt Lake City, UTAH (USA)
Small Medical Device Companies Can Now Manage and Automate their Design Control Process to Ensure Compliance with 21 CFR Part 820 with the Help of MasterControl's Design Control Software Systems at a Low Monthly Cost. Each manufactured medical device must be supported by appropriate documentation which demonstrates that its development followed the Food and Drug Administration's (FDA) design ...
Manufactured by:Stilla Technologies based inVillejuif, FRANCE
An increasing number of laboratories are using electronic records and electronic signatures for exchanging and storing data. Electronic documentation offers many benefits, including increased efficiency and productivity when storing data and easier information sharing and data mining. The Food and Drug Administration (FDA) of the United States (USA) regulates the food and drug industry of the USA ...
by:Evolucare Technologies Shenzhen Co., Ltd based inFutian District, CHINA
The multidisciplinary solution for your hospital information ...
Manufactured by:3DHISTECH Ltd. based inBudapest, HUNGARY
The CaseManager pathology management system gives the pathologist full control of the clinical workflow. CaseManager offers a comprehensive system from digitization through pathologist assignment, primary inspection, additional orders, and consultation to image analysis. CaseManager is the ultimate tool to access and handle all necessary information of the medical cases / that the medical ...