Show results for
Refine by
Clinical Data Software In France
10 software items found
Manufactured by:Novacor based inRueil-Malmaison, FRANCE
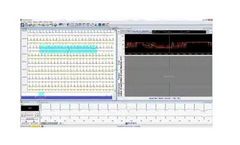
HolterSoft Ultima has been specifically developed to support the full range of Novacor Holter and ABPM recorders. It is fully optimised to provide an efficient analysis platform in busy clinical environments. Data is displayed logically and can be reviewed rapidly. Accurate, detailed and comprehensive reporting is almost instant. HolterSoft Ultima is fully ...
Manufactured by:Novacor based inRueil-Malmaison, FRANCE
RTSoft Ultima has been specifically developed to enable the unique features of the R.Test 4 to be fully and efficiently utilised in the clinical environment. Data is displayed logically and can be reviewed rapidly. Adaptive conclusion templates make detailed and comprehensive reporting almost instant. Built on the Microsoft .Net Framework to ensure security, ...
by:Intrasense based inMontpellier, FRANCE
Myrian® XP-Brain Perfusion CT is a hemodynamic perfusion imaging tool with three specific objectives: early detection of areas of hypoperfusion, determination of prognosis and evaluation of therapeutic efficacy. Its main indications are ...
by:SOPHiA Genetics based inBoston, MASSACHUSETTS (USA)
Powered by the advanced analytical capabilities of SOPHiA DDM™ Platform, this sample-to-report application determines HRD status of tumors, helping healthcare professionals increase their efficiency and confidence of getting actionable clinical insights and making data-driven decisions that improve the quality of patient ...
by:SOPHiA Genetics based inBoston, MASSACHUSETTS (USA)
Powered by the advanced analytical capabilities of SOPHiA DDM™ Platform, this sample-to-report application helps healthcare professionals increase their efficiency and confidence of getting actionable clinical insights and making data-driven decisions that improve the quality of patient ...
by:SOPHiA Genetics based inBoston, MASSACHUSETTS (USA)
Clinical interpretation has been one of the most complex and time-consuming aspects of transforming genomic data into meaningful results, until ...
by:Qynapse based inParis, FRANCE
The software is intended to be used by medical personnel or neuroimaging trained personnel to support diagnosis of central nervous system diseases. Available for research use only and protected by 3 patents, QyPredict has the potential to improve targeted patient selection in clinical trials and prediction of disease progression in patients. QyPredict is the future, and the future is ...
by:Novatek International based inMontreal, QUEBEC (CANADA)
In clinical studies, assessing and managing risks, as well as establishing areas of uncertainty that contribute to risk, are critical to a company’s decision making processes. Many business units within pharmaceutical, biotechnology and clinical research organizations must comply with federal regulations concerning their processes and computerized systems. One of the most challenging ...
by:SeqOne S.A.S. based inMontpellier, FRANCE
The integrated genomic analysis solution that delivers accurate results with increased efficiency and fast turnaround ...
Manufactured by:FeetMe Devices based inParis, FRANCE
FeetMe provides full mobility assessment with spatio-temporal parameters and plantar pressure at home and in clinic. FeetMe’s Evaluation application allows an easy and comprehensive data collection for a patient’s gait and activity parameters at home and in clinic. The richness of the data combined with a robust and ...