Refine by
Medical Software In Japan
52 software items found
by:CRScube Inc. based inSeoul, SOUTH KOREA
cubeCONSENT® provides a learning environment for subjects and ensures that the subject completely understands the trial prior to signing the Informed Consent Form (ICF). cubeCONSENT® is interoperable and integrated with the cubeCDMS® with subjects being automatically enrolled in cubeCDMS® upon signing the ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
cubePRO® is a mobile application used to collect ePRO (electronic Patient Reported Outcomes) data. Data is entered directly by participating patients which leads to a minimization in data reconciliation as the data is automatically synced with cubeCDMS®' database. cubePRO® is setup using cubeBUILDER® and can be equipped with edit checks which results in a reduction in errors and ...
by:TriNetX, LLC based inCambridge, MASSACHUSETTS (USA)
Our Key Opinion Leader (KOL) activity reports detail which physicians are treating which patients, by condition, drug, and provider specialty. Each physician in your customized report is identified by NPI, full name, mailing address, phone number, and email address when available. We generate our KOL activity reports from up-to-the-day, open claims data representing 1.8 million providers at ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
cubeLMS (Learning Management System) is a solution used for training and record management of internal personnel. cubeLMS features intuitive training scheduling, central real-time monitoring of training progress with metrics, viewing and exporting of training history for each staff/training course. cubeLMS also enables users to send announcements to specific users or teams to ensure timely ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
Pharmacovigilance requires a lot of resources such as personnel, processes, and management systems. cubeSAFETY® streamlines the Pharmacovigilance Reporting process; all the while being compliant with the regulations of regulatory agencies such as the FDA, EMA, CDE, MFDS, and ...
by:TriNetX, LLC based inCambridge, MASSACHUSETTS (USA)
TriNetX Open Claims data sets are built to close data gaps and provide insights throughout the product lifecycle, including market analysis and forecasting. Claims are sourced from clearinghouses that have processed more than 4 billion medical and pharmacy claims for over 300M patients since 2014; data is refreshed daily to enable an up-to-date understanding of market access and treatment ...
by:CRScube Inc. based inSeoul, SOUTH KOREA
cubeIWRS® is a powerful solution that manages all aspects of a study's randomization, drug distribution, and delivery logistics. With cubeIWRS®, users have an intuitive, single platform that oversees randomization and drug supply management. ...
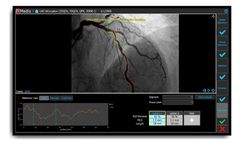
by:Medis Medical Imaging Systems B.V. based inLeiden, NETHERLANDS
The industry-leading, defacto standard and vendor-independent software solution for your coronary and ventricular X-Ray ...
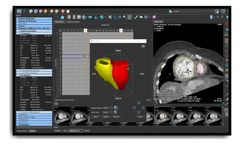
by:Medis Medical Imaging Systems B.V. based inLeiden, NETHERLANDS
The innovative, vendor-independent post-processing software solution for advanced quantitative analysis of cardiovascular CT ...
by:COGNITE AS based inLysaker, NORWAY
Take advantage of unmatched data management and comprehensive AI capabilities to improve operational performance, reduce costs, and unlock opportunities in ...
Manufactured by:TEAC Corporation based inTama-shi, JAPAN
Introducing a powerful content management solution that’s both easy to implement and affordable, with secure web-based access to all of your recorded surgical videos and images throughout a hospital’s network for quick searching, playback, organizing, editing and ...
Manufactured by:Agilent Technologies, Inc. based inSanta Clara, CALIFORNIA (USA)
The SureSelect Focused Exome is a highly targeted design that enables analysis of only the disease-associated targets providing deep coverage even on a benchtop sequencer. This design provides 20 or more reads for 95% of targets at 1.5Gb (100x) of sequencing and 98% of targets with 3Gb (200x) of sequencing enabling superior coverage of disease-associated regions even when sequenced on benchtop ...
Manufactured by:Medispec based inGaithersburg, MARYLAND (USA)
The MediConsole system is a computerized operating console that supports both the E3000™ and E2000™ Extracorporeal Shockwave Lithotripsy (ESWL) systems. Integrated with Visionspec™ system. Remote control of motorized treatment table, X-ray and lithotripter. Automatic stone localization. Remote operation allows for zero exposure, away from X-ray and electromagnetic field sources. ...
Manufactured by:smartData Enterprises Inc based inWhite Plains, NEW YORK (USA)
The current crisis has demonstrated the relevance of telehealth and created an opening to modernize the care delivery system. This modernization will be achieved by embedding telehealth in the care continuum at scale. A $3 billion revenue market has the potential to grow to $250 billion. The seeds for success will be sown in the next few months during the COVID-19 crisis. Healthcare systems that ...
Manufactured by:Hottinger Bruel & Kjær A/S based inVirum, DENMARK
Predict structural durability from major FEA results. nCode DesignLife is a CAE-based fatigue analysis system that contains a comprehensive set of solvers and advanced methods for predicting structural durability from major finite element analysis (FEA) result files. It identifies critical locations and calculates realistic fatigue lives for both metals and composites. Design engineers can go ...
by:TriNetX, LLC based inCambridge, MASSACHUSETTS (USA)
Trials that include patients in Japan have an advantage when it comes to regulatory approval in that country, where pharmaceutical sales reached 94B USD in 2020. But approval requires promising results from a well-executed study. Start the process the right way by understanding Japan's patient population, designing a feasible study, and selecting the right trial sites. ...
by:MasterControl, Inc based inSalt Lake City, UTAH (USA)
Small Medical Device Companies Can Now Manage and Automate their Design Control Process to Ensure Compliance with 21 CFR Part 820 with the Help of MasterControl's Design Control Software Systems at a Low Monthly Cost. Each manufactured medical device must be supported by appropriate documentation which demonstrates that its development followed the Food and Drug Administration's (FDA) design ...
by:TeraRecon, Inc. based inDurham, NORTH CAROLINA (USA)
TeraRecon is continually innovating to bring the industry the latest in AV capabilities! Intuition 4.6 includes Dual Monitor Support, Patient List Filtering, Glow Rendering, New Measurement Tools, HL7 Communication Protocol, and an all-new Neuro Perfusion ...
Manufactured by:smartData Enterprises Inc based inWhite Plains, NEW YORK (USA)
We deal with a new information technology generation that involves big data, internet of things (loT), cloud computing, and artificial intelligence to transform the conventional medical practices in an all-round way, making healthcare more efficient, more personalised, and more convenient. Smart healthcare services like smartHealth and smartTelehealth are utilising the technology that leads to ...
by:MasterControl, Inc based inSalt Lake City, UTAH (USA)
OOS Software to Automate Out of Specification (OOS) Processes to Comply with FDA CGMPs and 21 CFR Part 211 and Part 820 Regulations. In the FDA environment, specifications are essential in maintaining quality. CGMP regulations for finished pharmaceuticals (21 CFR Parts 210-211) and medical devices (21 CFR Part 820) require strict conformance to approved specifications which can be acheived easily ...