- Home
- Software
- north america
- medical devices software
Refine by
Medical Devices Software Software In North America
8 software items found
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
We are a regulated Software as a Medical Device (SaMD)-focused biotechnology company that develops our applications as medical treatments in accordance with all relevant FDA, ISO and IEC ...
by:SiteRocket Labs based inBoston, MASSACHUSETTS (USA)
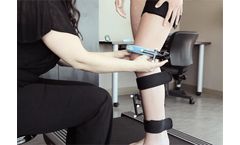
The challenge: Emovi develops and manufactures the KneeKG system, a medical device that assesses knee motion for patients with impaired movement due to an orthopedic cause. The company wanted to modernize some of its software and develop a cloud-based clinical portal without compromising security or reliability. Our solution: We modernized a ...
by:Quality Institute of America - QIA based inHouston, TEXAS (USA)
QIA offers a comprehensive suite of software modules designed for pharmaceutical and medical device companies to efficiently manage their Quality Management Systems. The software allows businesses to initiate tasks following best practice workflows and manage them through alerts and escalations. ...
Premium
Manufactured by:AB Sciex LLC based inFramingham, MASSACHUSETTS (USA)
Analyst MD Software: Rely on the Proven Performance for High-Quality Quantitation on your SCIEX IVD LC-MS/MS. Modern clinical laboratories are increasingly opting for mass spectrometry as their preferred sample analysis method. To aid the easy application of mass spectrometry, these labs need software that is intuitive and accessible to both novice and expert ...
by:Cox Prosight based inAtlanta, GEORGIA (US) (USA)
Cox Prosight offers a medical inventory tracking and forecasting software designed to provide real-time visibility into inventory levels and improve demand forecasting capabilities. This system helps to ensure essential medical equipment such as infusion devices, monitors, beds, stretchers, ventilators, and WoundVACs are readily ...
by:AQA Company, Inc. based inLa Canada, CALIFORNIA (USA)
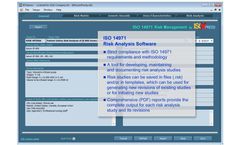
IMSXpress 14971 Medical Device Risk Management software is a Windows application for implementing Risk Analysis, Risk Evaluation, and Risk Control in strict compliance with the ISO 14971:2012 standard. Risk analysis, risk evaluation, and risk control methodologies strictly follow requirements of ISO 14971 and all recommendations included in ISO ...
Manufactured by:Nextern Inc based inWhite Bear Lake, MINNESOTA (USA)
We are your sole partner to provide seamless connected medical device development solutions. Leveraging our experience and expertise in medical device design, cloud architecture, and mobile engagement ...
by:BrightInsight, Inc based inSan Jose, CALIFORNIA (USA)
The leading global regulated Digital Health platform for biopharma and ...