- Home
- Software
- usa california
- medical devices
Refine by
Medical Devices Software In Usa California
12 software items found
by:Click Therapeutics, Inc based inNew York, NEW YORK (USA)
We are a regulated Software as a Medical Device (SaMD)-focused biotechnology company that develops our applications as medical treatments in accordance with all relevant FDA, ISO and IEC ...
by:AQA Company, Inc. based inLa Canada, CALIFORNIA (USA)
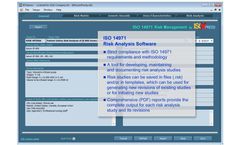
IMSXpress 14971 Medical Device Risk Management software is a Windows application for implementing Risk Analysis, Risk Evaluation, and Risk Control in strict compliance with the ISO 14971:2012 standard. Risk analysis, risk evaluation, and risk control methodologies strictly follow requirements of ISO 14971 and all recommendations included in ISO 14971 Annex B ...
Manufactured by:Coala Life Inc. based inIrvine, CALIFORNIA (USA)
Coala is a medical device that allows for long-term, non-invasive and algorithm driven cardiac monitoring. No patches, no burden, no limitations in time. Coala offers real- time, algorithm based detection of cardiac events including event notification. ...
by:Vintara based inOakland, CALIFORNIA (USA)
Vintara’s Enterprise Portal helps leaders deploy world class best practices for the manufacture and sale of medical devices. Our configurable workflow applications will drive performance and world class regulatory compliance including: ISO 13485, 21 CFR Part 11 compliance, 21 CFR Part 820 compliance, CAPA, Device Master Records, Customer ...
Premium
Manufactured by:AB Sciex LLC based inFramingham, MASSACHUSETTS (USA)
Analyst MD Software: Rely on the Proven Performance for High-Quality Quantitation on your SCIEX IVD LC-MS/MS. Modern clinical laboratories are increasingly opting for mass spectrometry as their preferred sample analysis method. To aid the easy application of mass spectrometry, these labs need software that is intuitive and accessible to both novice and expert ...
by:Novatek International based inMontreal, QUEBEC (CANADA)
Successful innovation programs more and more key to driving corporate growth and maintaining competitive advantage. Innovation programs must be sustainable and agile to keep pace with today’s rapidly changing environments. Companies struggle with how to establish and sustain the innovation process. Persons within the organization are an excellent resource for new ideas. Organizations ...
by:RapidAI based inSan Mateo, CALIFORNIA (USA)
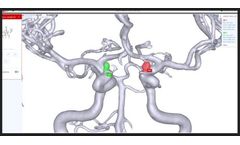
Rapid Aneurysm is an FDA-cleared imaging platform for cerebral aneurysm management. Helping physicians save time, improve patient care, and increase revenue by automating the process of finding, tracking, and proactively treating aneurysms. From a patient’s imaging scans, the platform creates a personalized 3D model of the vasculature and provides tools for accurate measurement and ...
by:DNAnexus, Inc. based inMountain View, CALIFORNIA (USA)
DNAnexus GxP Support ensures that your drug or device development work complies with best practice standards, at every ...
by:Veeva Systems Inc. based inPleasanton, CALIFORNIA (USA)
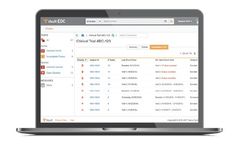
Vault EDC for clinical trials provides a fast and intuitive interface for capturing and reviewing data from sites. Eliminate constraints and run the trial you want with a modern EDC ...
by:BrightInsight, Inc based inSan Jose, CALIFORNIA (USA)
The leading global regulated Digital Health platform for biopharma and ...
Manufactured by:Carrot Medical based inBothell, WASHINGTON (USA)
Record Medical Cases for Review rchive Clinical Data ive Stream for Healthcare Educators. Live-stream the data from any medical device to any screen with ...
Manufactured by:Esaote S.p.A based inGenova, ITALY
With a web-based zero-footprint architecture, it is designed to be used as a universal viewer of diagnostic-quality medical images, supporting any device, without any loss of quality. Feature-rich, dedicated viewing protocols, multi-monitor support and possibility to view multi-mode images including breast tomosynthesis and echocardiography; allows the user to ...