- Home
- Software
- usa massachusetts
- clinical built support
Show results for
Refine by
Clinical Built Support Software In Usa Massachusetts
6 software items found
Manufactured by:Heidelberg Engineering Inc. based inFranklin, MASSACHUSETTS (USA)
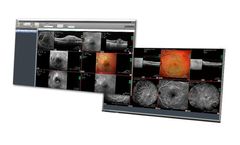
HEYEX PACS is the HEIDELBERG EYE EXPLORER solution for full integration of the Heidelberg Engineering ecosystem with third-party information sources. Streamline your workflow management by eliminating duplicated data entry and improving ...
by:N-SIDE based inLouvain-La-Neuve, BELGIUM
End-to-end clinical trial supply chain visibility and optimization. Enable ambitious clinical plans through an optimal supply ...
by:N-SIDE based inLouvain-La-Neuve, BELGIUM
Optimize end-to-end production planning from DS to IMP. The N-SIDE Production App gives you unprecedented flexibility to optimize manufacturing for your clinical projects, from drug substance (DS) to drug product (DP) to investigational medicinal product (IMP). Use the Production App to build and maintain an optimal clinical production plan that accounts for your specific constraints. Respond ...
by:SOPHiA Genetics based inBoston, MASSACHUSETTS (USA)
Clinical interpretation has been one of the most complex and time-consuming aspects of transforming genomic data into meaningful results, until ...
by:SOPHiA Genetics based inBoston, MASSACHUSETTS (USA)
Identifying patterns of genomic scarring in ovarian cancer samples. Accurate identification and reporting of Homologous Recombination Deficiency (HRD) status are critical for better patient management. SOPHiA DDM Dx HRD Solution is a CE-marked in vitro diagnostic (IVD) application leveraging low-pass Whole Genome Sequencing (WGS) and a proprietary deep-learning algorithm. Powered by the advanced ...
by:SOPHiA Genetics based inBoston, MASSACHUSETTS (USA)
Enabling novel fusion detection in small samples to improve lung cancer management. Sensitive novel fusion detection optimized for small lung cancer FFPE biopsies enhance patient care. SOPHiA DDM™ Dx RNAtarget Oncology Solution (ROS) is a CE-marked in vitro diagnostic (IVD) application based on next-generation sequencing (NGS) enabling accurate and sensitive detection of novel ...