Refine by
Drug Production Articles & Analysis
172 news found
Following regulatory approval, Advanced Instruments, a wholly-owned subsidiary within Patricia Industries, has now completed its acquisition of Nova Biomedical, a global provider of analytical instruments and consumables for the biopharmaceutical and clinical markets. The enterprise value amounts to USD 2.2bn. Patricia Industries has contributed USD 1.6bn in financing. The remainder of the ...
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, has announced the availability of its quality Purified Exosomes to support research in drug delivery, regenerative medicine, and various therapeutic applications. ...
An Abbreviated New Drug Application (ANDA) is an application submitted to the U.S. FDA to demonstrate that a generic drug is equivalent to a previously approved Reference Listed Drug (RLD) in terms of safety, efficacy, and quality. The ANDA contains information used for the review and approval of a generic drug ...
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, recently unveiled its significantly enhanced online ordering system. ...
Alfa Chemistry is one of the leading chemical manufacturers and suppliers who has been able to maintain its leadership position in the pharmaceutical sector by offering a broad product portfolio that caters to all aspects of the drug creation process. ...
With the evolution of pharmaceutical industry over the past decades, compliance remains a key factor in bringing novel drugs to market. Proregulations, as a product safety and regulatory consulting firm with a long-standing core mission of "Value in Compliance", announces to offer a series of DMF document production and filing services, helping ...
Specializing in complex biologic formulations, Biologics & Biosimilars service team of CD Formulation is revolutionizing how drug conjugate products are conceptualized and produced, standing out as a crucial ally in drug research and development. ...
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, is thrilled to announce the launch of its new line of liposomes specifically designed for food applications, offering promising delivery systems to enhance the bioavailability, stability, and taste of various food ingredients. ...
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, is proud to announce its expanded capabilities in Customized Liposomes. ...
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, has recently expanded its low PDI polymer portfolio and announces its new offering of Polyolefin Family polymers with a wide range of properties that make them well-suited for a variety of drug delivery applications. ...
Adial announced the publication of a peer-reviewed article highlighting the promising clinical results, strong safety profile and high compliance among patients administered AD04 (low-dose ondansetron), the Company's lead investigational new drug product being developed for the treatment of Alcohol Use Disorder (AUD). ...
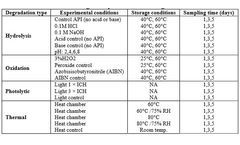
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announced the launch of its new Force Degradation Services to help pharmaceutical and medical device companies evaluate the stability of their drug candidates and finished products under a variety of stress conditions, ensuring their safety and efficacy throughout ...
BySTEMart
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, has announced an expanded portfolio of Polydimethylsiloxane (PDMS) products, empowering researchers with versatile and biocompatible tools for groundbreaking discoveries. ...
Understanding the solid-state properties of a compound can help researchers improve drug formulation, optimize manufacturing processes, and ensure the efficacy and safety of the final drug product. ...
We are delighted to announce that, thanks to our loyal customers, Cell Metric® X has been nominated in the Best New Drug Discovery & Development Product of 2023 category. Voting is now open, so if you’re happy with how this product has had an impact on your laboratory’s work, please take a moment to give us your support ...
About Tevogen Bio Tevogen Bio is driven by a team of highly experienced industry leaders and distinguished scientists with drug development and global product launch experience. Tevogen Bio’s leadership believes that accessible personalized immunotherapies are the next frontier of medicine, and that disruptive business models are required to sustain ...
Pharmaceutical excipients are inactive substances combined with active pharmaceutical ingredients (APIs) to create final drug products. These excipients play a crucial role in ensuring the stability, effectiveness, and safety of medications, as well as improving their palatability and appearance. ...
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, has launched a new line of Locust Bean Gum with advanced technical platforms to help scientists advance biopharmaceutical research. ...
In the wake of the COVID-19 pandemic, BOC Sciences, the world's reputed chemical supplier, declares that it will increase production to offer pharmaceutical impurity standards in bulk to satisfy unmet demands. Innovations in manufacturing processes, more complicated formulations, and increasingly complex global supply chains are just some of the factors making it more difficult ...
CD Bioparticles, a leading manufacturer and supplier of numerous drug delivery products and services, recently introduced a line of highly efficient mRNA Transfection Reagents with low toxicity for research applications, including the mRNA Transfection Reagent, Stem mRNA Transfection Reagent, and mRNA/gRNA Transfection Reagent. mRNA transfection is the process of ...