Medical Device Test Articles & Analysis
12 news found
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announces the expansion of its medical device testing capabilities and introduces Balloon Catheter Testing Services for the development of safe and effective medical ...
BySTEMart
The new service offerings include medical device safety testing, biocompatibility testing, validation and verification, and reusable medical device testing. ...
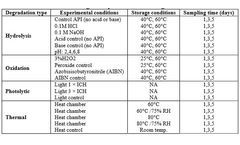
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announced the launch of its new Force Degradation Services to help pharmaceutical and medical device companies evaluate the stability of their drug candidates and finished products under a variety of stress conditions, ensuring ...
BySTEMart
STEMart, a provider of integrated medical device CRO services dedicated to medical device and diagnostic clinical development, now launches Pyrogenicity Testing service for the medical device industry. This new testing follows the biocompatibility guidelines ...
BySTEMart
Verification is critical for medical device testing, which is why we recommend reading part one if you haven’t already. In part one of this series, we examined the need for medical testing and briefly covered the first few aspects of device verification. ...
(OTCQB: GTHP), the maker of the LuViva™ Advanced Cervical Scan, based on its patented biophotonic technology, announced today its Chinese co-manufacturing partner and distributor for China, Shandong Yaohua Medical Instrument Corporation (SMI), has received notice from the Jinan Medical Device Quality Supervision and Testing ...
Attwill Medical. Solutions is one of the largest manufacturers of reagents for COVID-19 and other PCR testing kits, and. has unique freeze-drying technology, which may be utilized for the development of vaccines in a pill. form. In addition, they have produced many other lifesaving medical device technologies, and are. assisting ...
Pierre-Yves Frouin, BioSerenity CEO states, “Being a contracted provider with Premier furthers our commitment to bring world class medical devices and technological solutions to hospitals.” He also added, “BioSerenity is committed to improving patient care by making diagnostics more readily available. ...
The National Evaluation System for health Technology Coordinating Center (NESTcc), an initiative of the Medical Device Innovation Consortium (MDIC), announces twelve new Test-Cases which leverage Real-World Evidence (RWE) and address key priorities for medical device stakeholders. These ...
The lubricant has been added to the preventive maintenance plan for all of the medical devices involved in the recall. One company that has been instrumental in helping manufacturers of medical devices test their products and provide problem solving expertise is EMSL Analytical, Inc. EMSL’s ...
Stability testing using accelerated aging protocols shall be regarded as sufficient evidence for claimed expiry date until data from real time aging studies are ...
Scope 1.1 This specification provides a characterization of the design and mechanical function of external skeletal fixation devices (ESFDs), test methods for characterization of ESFD mechanical properties, and identifies needs for further development of test methods and performance criteria. ...