Surgical Mesh Laser Articles & Analysis
11 news found
BRIM Biotechnology, Inc. (“BRIM,” TPEx 6885) announced today that it has officially submitted the Phase 3 clinical trial protocol for BRM421 in the treatment of dry eye disease (DED) to the U.S. Food and Drug Administration (FDA). If the FDA has no further comments after the 30-day review period, BRIM will proceed to initiate the Phase 3 clinical trial in December. Based on its ...
Advanced Energy Industries, Inc. (Nasdaq: AEIS) - a global leader in highly engineered, precision power conversion, measurement and control solutions - today introduced the Excelsys FC1500, the industry’s first capacitor charger and multiple output power supply. The FC1500 delivers constant charge power and eliminates the need for multiple power supplies in medical laser and Intense Pulsed ...
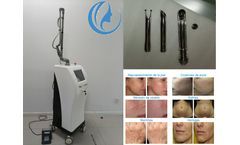
CO2 fractional laser resurfacing machine treatment can quickly improve skin texture, firm skin, improve large pores, and make skin smooth and tender like water. CO2 fractional laser technology is now the world's leading technology for wrinkle removal and skin rejuvenation. Through pulsed fractional laser, laser beauty has been raised to a new level. Bvlaser is a professional CO2 fractional laser ...
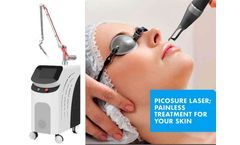
Today, let us take a look at picosecond laser machine. This machine is an upgraded version, which has special feature and advantage. If you are considering to buy a new picosecond laser machine for your salon or clinic, I think something you need to knowledge. Bvlaser is a CE certification picosecond laser FDA machine factory manufacturer, we can provide OEM/ODM picosecond laser service. How long ...
Valo Health, Inc. (“Valo”), the technology company focused on transforming the drug discovery and development process using human-centric data and artificial intelligence, today announced that dosing has commenced in its Phase 2 study — a multi-center ...
Pavilion Surgery Center, an affiliate of St. Joseph Hospital, along with Perimeter Medical Imaging AI, Inc. (TSX-V:PINK)(OTC:PYNKF) (FSE:4PC) (“Perimeter” or the “Company”) – a medical technology company driven to transform cancer surgery with ultra-high-resolution, real-time, advanced imaging tools to address high unmet medical needs – today jointly announced ...
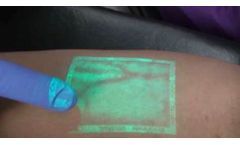
Kasuo Miyake, MD-PhD, is one of the world’s leading vascular surgeons, respected for his contribution to the development of new techniques for different types of varicose vein treatment. Based on his doctoral thesis, he created the CLaCS technique along with his father, and pioneered the use of augmented reality, like VeinViewer‘s near-infrared technology, in the treatment of micro ...
Business combination results in over $160 million in gross cash proceeds to Hyperfine Funds to catalyze commercial expansion of the world’s first FDA-cleared portable MRI systemTM and additional organic growth initiatives Hyperfine to begin trading on Nasdaq under ticker “HYPR” Hyperfine Inc., the groundbreaking medical device company that created Swoop®, the ...
BOSTON – September 30, 2021 — Activ Surgical™, a digital surgery pioneer, today announced that it has raised $45 million in a Series B financing round. The Series B round was led by Cota Capital, a multi-stage investment firm focused on private and public modern enterprise technology companies. Seven new investors, including BAM Funds, Magnetar Capital, MINT Venture ...
Easyfairs, owner of the renowned Aesthetics portfolio encompassing the UK’s leading events and publication in medical aesthetics, as well as the recently launched Beyond Beauty magazine, has today announced the launch of a new consumer-facing event, Beyond Beauty Live. The event will provide a platform for those interested in aesthetic treatments and cosmetic surgery to learn from medically ...
Injectsense, Inc. an innovative medical device firm that develops sensor-enabled digital health systems to measure therapy effectiveness, announced today additional members of its medical advisory board. The board, which includes world renowned specialists and surgeons in the ophthalmic field, provides valuable feedback on device requirements, procedures, risk mitigation, and regulatory issues. ...