- Home
- Applications
- USA
- clinical outcomes
Refine by
Clinical Outcomes Product Applications In Usa
11 applications found
A new paradigm, scientifically well established and recommended by WHO guidelines, relies on the introduction of the immune response as a new prognostic parameter to predict and refine the clinical outcomes of cancer patients. Maximize patient survival rates with an assessment of the tumor immune response. ...
ByVeracyte based in Marseille Cedex 9, FRANCE
Our flagship products, Virtue SAB and BackBeat CNT, have the potential to improve clinical outcomes and provide distinct commercial advantages within two of the largest and most well-established global medical device ...
ByOrchestra BioMed, Inc. based in New Hope, PENNSYLVANIA (USA)
Randomized clinical trials (RCT’s) have been shown significant improvement in clinical outcome when hyperthermia was added to radiotherapy and/or chemotherapy. In 2004 experts in clinical hyperthermia held a consensus meeting (Kadota Fund International Forum 2004) about the evidence of the published clinical results of the RCT’s. ...
ByPyrexar based in Salt Lake City, UTAH (USA)
FreeHold Duo / Trio Retractors are fully adjustable, hands-free, completely intracorporeal retractors that enable full surgeon autonomy throughout the procedure while optimizing visualization and exposure improving clinical ...
ByFreeHold Surgical, LLC based in New Hope, PENNSYLVANIA (USA)
Seamlessly integrate RPM datapoints into your existing protocols for patient care and maximize clinical ...
ByAthelas Incorporated based in Mountain View, CALIFORNIA (USA)
This program is designed to support research examining early resuscitation of patients presenting in shock using the LifeFlow PLUS device, a simple hand-held tool for rapid delivery of fluids or blood products. LifeFlow was developed by 410 Medical and received FDA clearance for crystalloid administration in 2016, with subsequent clearance for blood and blood products in May 2020. Many hospitals and EMS agencies across the U.S. have adopted LifeFlow for the initial management of a wide variety of conditions ...
By410 Medical based in Durham, NORTH CAROLINA (USA)
Gene expression is the most fundamental level at which the genotype gives rise to a phenotype. Whether you have RNA-Seq, qPCR or microarray data, Partek provides easy-to-use tools that guide you through the analysis process from start to finish within a point-and-click interface. With over 8,000 citations, see why our gene expression analysis tools are the most trusted in the genomics ...
ByPartek Incorporated based in Chesterfield, MISSOURI (USA)
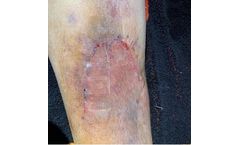
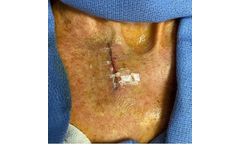
Non-invasive, topical skin closure device eliminates unnecessary “track marks” caused by sutures and ...
ByClozex Medical, Inc. based in Watertown, MASSACHUSETTS (USA)
Non-invasive, topical skin closure device eliminates unnecessary “track marks” caused by sutures and staples(1,4). Reduces scar-producing tension by evenly distributing forces along the entire length of the wound edges(1,4). Interlaced technology aligns and securely maintains skin edges in the same planar level, promoting optimal ...
ByClozex Medical, Inc. based in Watertown, MASSACHUSETTS (USA)
Save 70% of your time and maximize the probability of success of your clinical study. Avoid common pitfalls by starting your cohort study, patient registry, observational data collection, or clinical trial on Jeeva eClinical cloud platform. Save over 70% of the effort involved in toggling between numerous tools, coordination between multiple channels of engagement between study team and participants, participants use their own mobile device, and study specific data governance. ...
ByJeeva Informatics Solutions Inc. based in Manassas, VIRGINIA (USA)
Increase clinical translation with Organ-Chips for ADME-Tox assessments. Accurate ADME-Tox profiling of preclinical drug candidates remains a challenge. Preclinical ADME-Tox (Absorption, Distribution, Metabolism, Excretion, and Toxicity) studies are pivotal to predicting human response. However, approximately 30% of drugs fail during human clinical trials due to adverse reactions despite promising preclinical study results, and another 60% fail due to lack of efficacy. As companies broaden the modality toolbox ...
ByEmulate based in Boston, MASSACHUSETTS (USA)