Refine by
Locations
- Africa
- Algeria
- Angola
- Benin
- Botswana
- Burkina Faso
- Burundi
- Cameroon
- Cape Verde
- Central African Republic
- Chad
- Comoros
- Congo
- Congo, Democratic Republic Of The
- Djibouti
- Egypt
- Equatorial Guinea
- Eritrea
- Ethiopia
- Gabon
- Gambia
- Ghana
- Guinea
- Guinea-Bissau
- Ivory Coast
- Kenya
- Lesotho
- Liberia
- Libya
- Madagascar
- Malawi
- Mali
- Mauritania
- Mauritius
- Morocco
- Mozambique
- Namibia
- Niger
- Nigeria
- Rwanda
- Senegal
- Seychelles
- Sierra Leone
- Somalia
- South Africa
- St. Helena
- Sudan
- Swaziland
- SãO Tomé & PríNcipe
- Tanzania
- Togo
- Tunisia
- Uganda
- Zaire
- Zambia
- Zimbabwe
Circulating Dna Equipment & Supplies In Africa
18 equipment items found
Manufactured by:Omega Bio-tek, Inc. based inNorcross, GEORGIA (US) (USA)
The Mag-Bind cfDNA Kit is designed for rapid and reliable isolation of circulating DNA from 500-10,000 µL plasma or serum samples. The Mag-Bind® cfDNA Kit can be processed manually or on an automated platform. The procedure eliminates the need for funnels and vacuum steps, providing hands-free operation in automated ...
Manufactured by:Twist Bioscience based inSouth San Francisco, CALIFORNIA (USA)
NGS-based liquid biopsy is a rapidly evolving application that requires accurate and precise reference standards. The Twist Pan-cancer Reference Standard is a high-quality, standardized control for use in the research and development of NGS-based liquid biopsy assays. This reference standard is ideal for establishing the analytical limit of detection (LoD) for specific cancer variants and as a ...
Manufactured by:Guardant Health, Inc. based inRedwood City, CALIFORNIA (USA)
Our Guardant Reveal™ test is the first blood-only test that detects residual and recurrent disease in two weeks, without the need for a tissue biopsy. The test detects circulating tumor DNA (ctDNA) in blood after surgery to identify patients with residual disease who may benefit most from adjuvant therapy. Our first indication is early-stage colorectal ...
Manufactured by:Sysmex Partec GmbH based inGoerlitz, GERMANY
Highly sensitive down to 0.06 % MAF, Able to detect 6 MM with 95 % confidence across all mutations (absolute quantification), High flexibility of between 2-16 samples/run, Fast TAT (2 days), Convenient software. Plasma-SeqSensei (PSS) BC RUO Kit allows for the highly sensitive and specific detection of mutations in circulating tumour DNA (ctDNA) from the plasma ...
Manufactured by:Nucleix Ltd. based inSan Diego, CALIFORNIA (USA)
Lung cancer remains a leading cause of death from cancer globally, due to its high incidence and late-stage diagnosis. Most patients are currently diagnosed with late-stage cancer, though research has shown 5-year survival rates are up to 10 times greater when disease is detected early. Annual imaging (low-dose CT scans) is recommended for screening individuals with highest risk, but less than ...
Manufactured by:Rarecells Diagnostics SAS based inParis Cedex 06, FRANCE
Cancer is the second cause of death, accounting for nearly 9 million deaths in 2017 all over the world. In 2019, 1,762,450 new cancer cases and 606,880 cancer deaths are projected to occur in the United States. It is estimated that metastasis is the primary cause of 90% of all cancer-related deaths. Circulating Tumor Cells (CTCs) detection and analysis are the foundation of non-invasive tests ...
Manufactured by:OncoDNA S.A. based inGosselies, BELGIUM
OncoFOLLOW: The first personalised liquid biopsy in the market. Monitor the progression and detect lack of response. OncoFOLLOW is a test based on the analysis of circulating tumor DNA in blood. It is used to monitor the progression of the tumor (burden of the disease) and to detect lack of response or resistance to treatment as soon as it appears. This assay is ...
Manufactured by:Nucleix Ltd. based inSan Diego, CALIFORNIA (USA)
Liquid biopsies are a new powerful tool in cancer diagnostics that is transforming patient healthcare. Body fluids such as blood or urine are obtained from the patient and analyzed for the presence of circulating tumor DNA which is shed from the cancer. This DNA can tell if cancer is present, its type, effective medications, and more. Liquid ...
Manufactured by:Biocept based inSan Diego, CALIFORNIA (USA)
Biocept’s Target Selector™ molecular assay kits detect key oncogene mutations through the analysis of both Formalin-Fixed Paraffin-Embedded (FFPE) tissue gained from surgical biopsies as well as circulating tumor DNA (ctDNA) gained from blood-based liquid biopsies. The EGFR pathway can include mutations that are among the most frequently evaluated ...
Manufactured by:ViennaLab Diagnostics GmbH based inVienna, AUSTRIA
A reliable tool to personalize therapy of lung cancer patients with acquired TKI-resistance mutation T790M in the EGFR ...
Manufactured by:Datar Cancer Genetics based inGuildford, UNITED KINGDOM
Cancertrack is an advanced non-invasive blood test which provides real time intel on the status of a malignancy from a safe, simple and quick blood draw. Cancertrack profiles molecular features in circulating tumor DNA and circulating tumor RNA and also evaluates ‘Circulating Tumor Cells’ ...
Manufactured by:Biocept based inSan Diego, CALIFORNIA (USA)
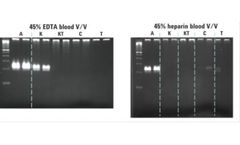
As healthy and diseased tissue interact with the bloodstream, they release both cellular and genetic material into the circulation. These materials are referred to as “cell-free” DNA (cfDNA), and circulating tumor cells (CTCs). Conventional blood collection tubes contain only anti-coagulants, designed to prevent blood clotting. Over ...
Manufactured by:Yourgene Health based inManchester, UNITED KINGDOM
The Sage™ Prenatal Screen is a non-invasive prenatal screening solution to estimate the risk of a fetus having Trisomy 21, Trisomy 18, Trisomy 13, Rare Autosomal Aneuploidies (RAA), Sex Chromosome Aneuploidies (SCA) and the most clinically relevant ...
Manufactured by:IMB Dx, Inc. based inSeoul, SOUTH KOREA
Cell-free DNA (cfDNA) is circulating fragments of DNA that is released from cells into the circulatory system throughout the body. Tumor cells also release DNA into the blood stream, which is called circulation-tumor DNA(ctDNA). IMBdx Inc.’s AlphaLiquid® platform offers industry-leading ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Signatera is a personalized, tumor-informed assay optimized to detect circulating tumor DNA (ctDNA) for molecular residual disease (MRD) assessment and recurrence monitoring for patients previously diagnosed with cancer, with broad utility for cancer ...
Manufactured by:Agilent Technologies, Inc. based inSanta Clara, CALIFORNIA (USA)
The SureDirect Blood PCR Kit enables direct DNA amplification from many sample types including: liquid blood, dried blood on paper, serum, and plasma. Inhibitor resistance and throughput, common PCR challenges associated with blood samples, are averted through the activity of a newly engineered Taq. Your workflow is streamlined by eliminating the cumbersome DNA extraction step. SureDirect Blood ...
Manufactured by:OncoDNA S.A. based inGosselies, BELGIUM
The Liquid Biopsy Solution For Disease Monitoring Or Therapeutic Decision. OncoSELECT is a fast and minimally invasive analysis of circulating tumor DNA from a blood sample for lung (NSCLC), colorectal and breast (HR+ or HER2+) cancer patients. It is the perfect solution to identify therapeutic options for cancer patients not able to have their tumor biopsied or ...
Manufactured by:IMB Dx, Inc. based inSeoul, SOUTH KOREA
Tissue specimens are either from surgical resections or biopsies which may raise the risk of bleeding, and infection and further require recovery from the procedure. Genomic profiling of tissue samples provides a single point in space and time which is lacking the ability to reveal tumor heterogeneity and to test repeatedly to monitor the treatment efficacy. The risk of tissue biopsy is much ...