- Home
- Equipment
- asia middle east
- bone void
Show results for
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Bone Void Equipment & Supplies In Asia Middle East
37 equipment items found
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
High purity Type-I collagen derived from bone is essential for tissue regeneration and remodeling in any osseous defect. Osseograft / DMBM (xenograft) is one such de-mineralized bone derived Type-I collagen for bone void filling ...
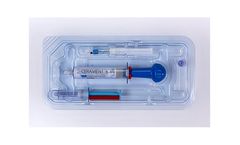
Manufactured by:BONESUPPORT AB based inLund, SWEDEN
CERAMENT BONE VOID FILLER is an injectable, moldable and drillable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the radio-contrast agent ...
Manufactured by:EnColl Corporation based inFremont, CALIFORNIA (USA)
Over 100 years of research, experimentation and use has demonstrated that calcium sulphate enhances bone regeneration and is totally biocompatible and and resorbable. At the same token, high purity type-I collagen DMBM (xenograft) also is essential for tissue regeneration and remodeling. Osseomold consists of both materials admixed in proper proportions to give the best biological and mechanical ...
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
CERAMENT BONE VOID FILLER is an injectable, moldable and drillable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the radio-contrast agent iohexol. The unique ratio of hydroxyapatite and calcium sulfate is designed to enable CERAMENT to resorb at the same rate that bone ...
Manufactured by:BONESUPPORT AB based inLund, SWEDEN
CERAMENT V is an injectable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the antibiotic vancomycin ...
Manufactured by:BONESUPPORT AB based inLund, SWEDEN
CERAMENT G is an injectable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the antibiotic gentamicin ...
Manufactured by:Leader Biomedical Group based inAmsterdam, NETHERLANDS
Cancellous and cortical bone chips with sound structural and mechanical properties for filling of bone loss and/or correcting of ...
Manufactured by:CAM Bioceramics B.V. based inLeiden, NETHERLANDS
Porous blocks with interconnected pores that are mainly used as raw material for manufacturing bone void fillers. Macropores, with diameters of approximately 600 µm, enable cell attachment, and the open interconnected porosity enables blood circulation. This specific morphology allows faster resorption of the ceramic material and accelerates ...
Manufactured by:Biedermann Motech GmbH & Co. KG based inVillingen, GERMANY
Sophisticated technology for fixation of bone fractures and bone reconstructions of the humerus. Can be utilized to deliver injectable bone void fillers to a surgical site. Compatible with MDS humerus plating systems. Innovative option for additional stability in osteoporotic and complex ...
Manufactured by:Surgentec LLC based inBoca Raton, FLORIDA (USA)
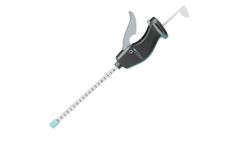
Surgentec brings a new advancement to the bone graft delivery arena with the Graftgun. Our Graft Delivery System (GDS®) is designed to allow for universal, quick, and accurate bone graft delivery to a surgical site without the problems of a traditional funnel.Its patented, controlled delivery method is designed to safely dispense bone graft ...
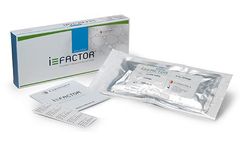
Manufactured by:Cerapedics, Inc. based inWestminster, COLORADO (USA)
i-FACTOR Bone Graft products are intended to replace or augment the use of autologous bone commonly utilized in spine, trauma, and orthopedic procedures such as: Spinal fusion incorporating interbody fusion devices; Treatment of non-union or traumatic fresh fractures; Joint reconstruction requiring a bone void filler. i-FACTOR ...
Manufactured by:EOS MEDICAL SOLUTIONS (EOSMED) based inSofia, BULGARIA
Biongraft is an injectable and formable paste bone graft based on hydrogel and Beta-tricalcium phosphate (β-TCP), including ZrO2 nanoparticles for antibacterial e?cacy. Biongraft is a safe and fully biocompatible material which is designed to act as an osteoconductive sca?old to support the ingrowth and fusion of adjacent viable bone when placed in an ...
Manufactured by:SYMATESE based inCHAPONOST, FRANCE
COLLAPAT®II is a collagen medical device containing hydroxyapatite used as bone substitute in orthopaedics, spine surgery and in maxillo facial surgery and odonto-stomatology. COLLAPAT® II is a bone void filler presented in a sponge form. It is composed of a collagen structure in which ceramised hydroxyapatite granules are dispersed. ...
Manufactured by:Medosis based inSofia, BULGARIA
Biongraft Dental Injectable Putty is a vital innovation in the field of bone grafting, primarily developed for dental applications. It is composed of a hydrogel and Beta-tricalcium Phosphate (β-TCP) matrix, enhanced with ZrO2 nanoparticles to provide antibacterial properties. The material serves as an osteoconductive scaffold, facilitating the growth and fusion of ...
Manufactured by:Graftys based inAIX EN PROVENCE Cedex 3, FRANCE
Graftys QuickSet is a Calcium Phosphate Cement of high viscosity with mechanical properties similar to those of cancellous bone, supplied in a user-friendly double-syringe, which is pre-filled with a powder (calcium phosphate salts and HydroxyPropylMethylCellulose (HMPC)) and with a phosphate-based (Na2HPO4) aqueous solution. Mixing of these two components in the syringe results ...
Manufactured by:Safeguard Medical based inHuntersville, NORTH CAROLINA (USA)
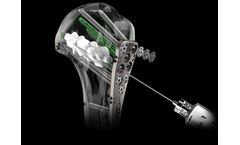
The NIO Infant™ provides safe, rapid intraosseous access in the proximal tibia as an alternative to IV access in emergency medical situations. The NIO Infant is a manual IO device with an innovative Stepped Needle® which provides graduated penetration into the bone marrow cavity to help prevent over-penetration. Additionally, the NIO Infant includes a Fixation dressing, which secures ...
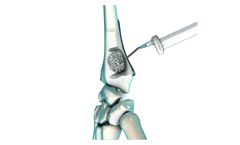
Manufactured by:Cervos Medical based inMarshfield, MASSACHUSETTS (USA)
The CER-SUB-1560 is a bone access device that has a 15 gauge cannula with an open tip and is designed to be powered by a drill. The device can serve as an access port to transplant tissue or medications. For example, marrow aspirate obtained using CER-EXT can be used with the CER-SUB-1560 to transplant marrow aspirate. The mechanism your body uses to repair and maintain tissue is Vasculogenesis, ...
Manufactured by:Osteogenics Biomedical based inLubbock, TEXAS (USA)
Zcore™ is an osteoconductive, porous, anorganic bone mineral with a carbonate apatite structure derived from porcine cancellous bone. Proprietary processing steps preserve both interconnecting macroscopic and microscopic porous structures of Zcore™ that helps support the formation and in-growth of new bone. ...
Manufactured by:Osteogenics Biomedical based inLubbock, TEXAS (USA)
Zcore™ is an osteoconductive, porous, anorganic bone mineral with a carbonate apatite structure derived from porcine cancellous bone. Proprietary processing steps preserve both interconnecting macroscopic and microscopic porous structures of Zcore™ that helps support the formation and in-growth of new ...
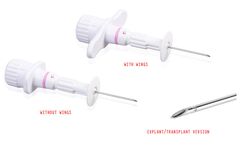
Manufactured by:Medax Srl based inSan Possidonio (MO), ITALY
Bone Marrow aspiration system “XS”, also available in the explant/transplant version ...