- Home
- Equipment
- australasia
- venous system
Show results for
Refine by
Applications
- Neuromuscular Electrostimulation Device for VTE Prevention – Obstetrics - Hospital Applications
- Neuromuscular Electrostimulation Device for Post-Operative Oedema Prevention - Hospital Applications
- Neuromuscular Electrostimulation Device for VTE Prevention - Acute Stroke- Hospital Applications
- Neuromuscular Electrostimulation Device for Pre-operative Oedema Reduction - Ankle Fracture - Hospital Applications
- Neuromuscular Electrostimulation Device for Post-operative Oedema Reduction - Hip Replacement - Hospital Applications
- Neuromuscular Electrostimulation Device for VTE Prevention – NICE Guidance- Hospital Applications
Venous System Equipment & Supplies In Australasia
28 equipment items found
Manufactured by:Fisioline S.r.l. based inVerduno, ITALY
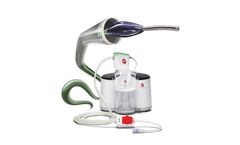
This type of device allows acting physiologically wherever helping for problems due to an incorrect functioning of the lymphatic or venous system is needed. LINFOPRESS MASTER exploits a powerful and silent compressor in order to obtain inflating cycles with an accurate pressure regulation (0÷200 mmHg) in every single sector, constantly displayed on a wide ...
Manufactured by:Healthline Medical Products based inSalt Lake City, UTAH (USA)
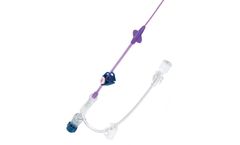
The ORION™ Central Venous Catheter is designed for short-term (less than 30 days) access to the central venous system for intravenous administration. This non-tunneled CVC is available in a variety of sizes and lengths to give clinicians multiple options to meet patients’ ...
Manufactured by:Healthline Medical Products based inSalt Lake City, UTAH (USA)
The Health Line CT Midline Catheter is intended for short-term (less than 30 days) peripheral access to the venous system for the purpose of intravenous therapy, medicines, and blood products. A single midline IV access can meet infusion therapy requirements for patients needing 5 to 7 days of therapy, eliminating multiple needle sticks and enhancing patient ...
Manufactured by:Penumbra, Inc. based inAlameda, CALIFORNIA (USA)
Penumbra’s Indigo Aspiration System can be used to remove emboli and thrombi from vessels of the peripheral arterial and venous systems, and for treatment of pulmonary embolism. A minimally-invasive device, Indigo enables the restoration of blood flow in such cases as acute limb ischemia and venous thrombus. The Indigo ...
Manufactured by:United Therapeutics Corporation based inSilver Spring, MARYLAND (USA)
The most widely recognized method of removing cells in use today is “immersion decellularization” in which a tissue or organ is soaked in strong detergent which diffuses from the outer surface inward, and then back out once the cells are dissolved which is limited to a few millimeters. The end result is a partially degraded scaffold with a compromised vascular network and an outer ...
Manufactured by:medi GmbH & Co. KG based inBayreuth, GERMANY
Experience compression treatment and unparalleled comfort in wear. With mediven sheer & soft, only you will know that you’re wearing a compression stocking. But you’ll certainly feel the ...
Manufactured by:Baylis Medical Company, Inc based inMontreal, QUEBEC (CANADA)
Complex Diagnostic Mapping Solutions Go Further. Know More. Enhanced diagnostic precision for coronary sinus mapping and ...
Manufactured by:Access Vascular, Inc. based inBillerica, MASSACHUSETTS (USA)
A midline catheter is a peripheral line used to gain vascular access with the internal tip located level at or near the level of the axilla and distal to the shoulder. ...
Manufactured by:Nuwellis based inEden Prairie, MINNESOTA (USA)
The Aquadex SmartFlow System is FDA cleared to simply, safely, and precisely remove excess fluid (primarily salt and water) from patients suffering from fluid overload who have not responded to medical management, including diuretics. CLINICALLY PROVEN SOLUTION FOR TREATING FLUID OVERLOAD: Providers can specify and adjust the rate of fluid removed for each individual patient, resulting in a ...
Manufactured by:Access Vascular, Inc. based inBillerica, MASSACHUSETTS (USA)
A peripherally inserted central venous catheter (PICC) is a central line used to gain vascular access with its tip extending to the superior vena ...
Manufactured by:URGO Group based inParis, FRANCE
UrgoK2/ UrgoKTwo is an innovative multi-component technology that guarantees the reliable and continuous compression patients need to heal from their venous leg ...
Manufactured by:Bentley InnoMed GmbH based inHechingen, GERMANY
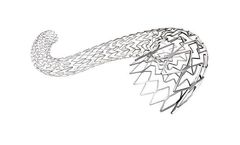
Thrombosis is known to be a wide spread disease. Venous stenting is proven to have a positive impact on the quality of life, especially of young and active people. The BeYond venous stent is designed to respect the natural venous anatomy. It offers the optimal balance between radial force, flexibility and deployment ...
Manufactured by:Baylis Medical Technologies based inMississauga, ONTARIO (CANADA)
This guidewire has been effectively employed to navigate and cut soft tissue structures within the cardiovascular, portal venous, biliary, urinary systems, and the gastrointestinal tract. With a rounded, atraumatic tip, it delivers RF energy to ensure controlled punctures with minimal collateral damage to surrounding tissues. The device has a maximum outer ...
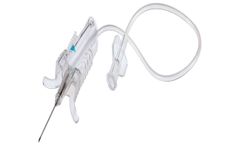
Manufactured by:KANGJIAN Medical Apparatus Co., Ltd. based inTaizhou City, CHINA
Model: Double-wing Safe type. Brand: KANGJIAN. Numbering: 0201-2122G. other: Please specify the specific requirements in the order ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
Sample with Confidence: A used needle can cause a needlestick injury, even if it is only exposed momentarily before being shielded. This can cause anxiety for healthcare professionals and patients alike. By utilising a user-centric design that provides protection once the needle is removed from the vein, Unistik® ShieldLock allows HCPs to draw large volumes of blood with ease and helps to ...
Manufactured by:TauroPharm GmbH based inWaldbuttelbrunn, GERMANY
We offer a variety of lock solutions that meet the individual needs of different patients. NutriLock™ has proven especially suitable in the context of cancer treatment and parenteral nutrition. Thanks to the antimicrobial agent taurolidine, it protects patients from catheter-related ...
Manufactured by:Owen Mumford Ltd. based inOxfordshire, UNITED KINGDOM
Sample with Confidence: A used needle may cause a needlestick injury, even if it is only exposed momentarily before being shielded. This may cause anxiety for healthcare professionals and patients alike. With its one-handed, in-vein activated safety shield, Unistik® ShieldLock Ultra helps to minimise the risk of needlestick injuries and give peace of mind for ...
Manufactured by:Amed Advanced Medication Co., Ltd. based inNew Taipei City, TAIWAN
This product provides reproducible and prolonged access to the venous vascular system and can be used to administer chemotherapy, antibiotics and antivirals, intravenous nutrition, collection of blood samples and transfusions or blood products. The product is composed of a catheter connected to a radiopaque infusion set and fixed by a connecting ring. ...
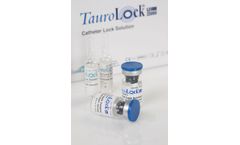
Manufactured by:TauroPharm GmbH based inWaldbuttelbrunn, GERMANY
We have developed catheter lock solutions for patients in various contexts. TauroLock™-U25.000 provides a particularly strong prophylaxis against occlusion, as urokinase serves to break up blood ...
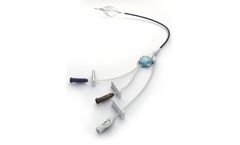
Manufactured by:Mermaid Medical Group based inStenløse, DENMARK
The Angel Catheter is an IVC filter permanently attached to a triple lumen central venous catheter. The smart, elegant design of the Angel Catheter provides critical care physicians and their patients with prophylactic PE protection and guarantees IVC filter retrieval, a game changing promise that no other IVC filter can make. As the first and only IVC filter to receive FDA clearance for a ...