Refine by
Applications
- Biomaterials for Spine Surgery
- Biomaterials for Trauma Surgery
- Solutions for Pharmaceutical
- Microbial Reduction Solutions for Cosmetic and Packaging
- Flagship products for therapeutic industry
- Low-Level-Laser Therapy for General
- Indications LLLT Application
- Artificialiris Medical Device for Patient
- Home Sleep Testing for Medical Professionals
- Home Sleep Testing for Sleep Apnea
- Biomaterials for Diabetic Foot Osteomyelitis Surgery
- Biomaterials for Mastoid Surgery
- Biomaterials for Benign Bone Tumor Surgery
- IONICON PTR-TOF instruments deployed for COVID-19 detection in breath
- Medical Device Solutions for Full Lifecycle
- Medical Devices for Moderate to Severe Heart Failure Treatment
- Medical Devices for Severe or Advanced Stage Heart Failure Treatment
- Medical Devices for Causes Heart Failure
- Medical Devices for Heart Failure Symptoms
- Medical Devices for Diagnosis and Evaluation
- Technology for Surface Modification and Drug Delivery
- Neuromuscular Electrostimulation Device for VTE Prevention – Obstetrics - Hospital Applications
- Neuromuscular Electrostimulation Device for Post-Operative Oedema Prevention - Hospital Applications
- Neuromuscular Electrostimulation Device for VTE Prevention - Acute Stroke- Hospital Applications
- Medical Device Solutions for Opportunity Realization
- Multi-Function Ventilator for Healthcare Professionals
- Solutions for Glaucoma and Intraocular Pressure (IOP)
- Medical Device for Clinicians
- Medical Device for Patients
- Solution for Ultrafast Laser Spectroscopy / Imaging
- Solution for High-Speed 4D Imaging
- Home Sleep Monitor for Clinical Validation
- Neuromuscular Electrostimulation Device for Pre-operative Oedema Reduction - Ankle Fracture - Hospital Applications
- Wound Therapy Applications Device for Mixed Aetiology Leg Ulcers
- Wound Therapy Applications Device for Venous Leg Ulcers
- Neuromuscular Electrostimulation Device for Post-operative Oedema Reduction - Hip Replacement - Hospital Applications
- Neuromuscular Electrostimulation Device for VTE Prevention – NICE Guidance- Hospital Applications
- Biomaterials for Bone Infection Surgery
- Brain Computer Interface Technology for Emagine Stroke Recovery
- Medical Devices for Medical Professionals
- Medical Devices for Patients
- Medical Devices for Chronic Venous Insufficiency: Eliminating Pain
- Medical Devices for Breast Cancer-Related Lymphedema
- Medical Devices for Lipedema
- Ultrasound measurement instrumentation solutions for diagnostic sector
- Ultrasound measurement instrumentation solutions for physiotherapy sector
Medical Device Equipment & Supplies In Cuba
1,688 equipment items found
Manufactured by:BioScience GmbH based inDümmer, GERMANY
Hyadent BG is a medical device intended for single use only and is produced from a hyaluronic acid of non-animal origin. ...
Manufactured by:Huons Medicare based inBusan, SOUTH KOREA
Purpose of use: sterilization of small spaces. Size (W*D*H): 500*20*495mm. weight: 19.5kg. voltage: 220V (60Hz) Sterilization of small spaces: Up to 150? ...
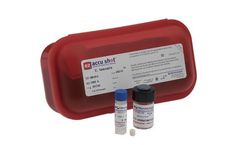
Manufactured by:Microbiologics based inSt. Cloud, MINNESOTA (USA)
Let’s face it; Growth Promotion Testing of culture media is likely not at the top of your "favorite lab activities" list. We get it, which is why we created EZ-Accu Shot. It provides a fast, convenient and reliable solution for Growth Promotion Testing, Media Challenge Testing and more. Each instant-dissolve microorganism pellet is designed to deliver 10-100 CFU per inoculum and provides 8 ...
Manufactured by:Angionica Ltd. based inLodz, POLAND
AngioExpert is a medical device which uses the FMSF-PORH method to evaluate microvascular circulation. ...
Manufactured by:Avania based inBrunswick, AUSTRALIA
Medical device clinical trials require highly specialized knowledge and a customized approach. Avania has experience across all medical device classifications. Our team has more than 30 years of experience managing successful medical device clinical trials across more than 30 countries. ...
Manufactured by:ASKION GmbH based inGera, GERMANY
The product development of medical devices necessitates a well structured approach. Reliability and safety of the final product is of paramount concern. With ASKION, you profit from our experience starting from specification to serial production and know your product in good hands. ...
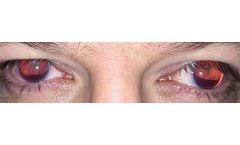
Manufactured by:VEO Ophthalmics, LLC based inCincinnati, OHIO (USA)
Managing congenital aniridia and acquired iris defects — including, but not limited to, traumatic iris defects and traumatic mydriasis — is often challenging. When iris reconstruction is necessary, the CUSTOMFLEX® ARTIFICIALIRIS is a unique device that offers both surgeons and patients important ...
by:MC10 based inLexington, MASSACHUSETTS (USA)
BioStamp nPoint is an FDA 510(k) cleared medical device designed to collect medical grade, clinical quality bio-metric, physiological and eCOA data in a clinical trial ...
Manufactured by:Pierrel S.p.A., based inCapua (CE), ITALY
Goccles is a medical device made in Italy developed by the Catholic University of Rome, that uses the common curing light present in every dental office to perform a quick and non-invasive examination of the autofluorescence of the oral cavity, identifying any precancerous and cancerous ...
Manufactured by:Plasma Surgical, Inc. based inRoswell, GEORGIA (US) (USA)
PlasmaJet delivers effective control to surgeons: Allows enhanced visualization, opening, and dissection of tissue planes. Controlled tissue effect enables use on or around sensitive structures, leading to more complete disease removal. Less than 0.3 mm depth of thermal effect on key structures such as bowel, ovary, and fallopian tubes. Less than 0.5 mm lateral thermal spread. Sealing of small ...
by:Qualityze Inc based inTampa, FLORIDA (USA)
Safety and quality are non-negotiable in the medical devices industry, that’s why we developed ISO 13485. Regulatory requirements are increasingly stringent throughout every step of a product’s life cycle, including service and delivery. Increasingly, organizations in the industry are expected to demonstrate their quality management processes and ...
Manufactured by:Beldimed s.a. based inAndrimont, BELGIUM
Using our know-how in human medicines, we decided to extend our activities to the field of medical ...
Manufactured by:Bio Molecular Systems based inUpper Coomera, AUSTRALIA
The world’s first magnetic induction cycler is now a registered medical device with CE-IVD and TGA approval. Mic IVD is manufactured under an ISO 13485:2016 Quality Management System. ...
Manufactured by:BMP Medical based inSterling, MASSACHUSETTS (USA)
Intravenous (IV) medical devices are one of the most technically challenging product lines to produce. Most IV device parts are very small and have extremely tight specifications and tolerances. BMP Medical utilizes it’s expertise in statistical process control, automation, decoupled molding processes, and quality ...
Manufactured by:Bimaks Chemicals Food Foreign Trade Ltd based inAnadoluhisari, TURKEY
Glutaraldehyde solution ; activated, and ready to use sporicide and sterilizing disinfectant containing 2% glutaraldehyde. Used for cold disinfection of medical ...
Manufactured by:Tristel Solutions Ltd. based inSnailwell, UNITED KINGDOM
Duo Wipes are the perfect partner for Tristel Duo OPH and Tristel Duo ULT. Duo Wipes are dry wipes made of a soft, durable, low-linting fabric that will not leave a buildup of lint on medical devices. Each wipe is low absorbing to ensure the maximum amount of foam is distributed onto the medical ...
Manufactured by:Bimaks Chemicals Food Foreign Trade Ltd based inAnadoluhisari, TURKEY
Ready to use device disinfectant with sporicide and sterilizing ...
Manufactured by:Bimaks Chemicals Food Foreign Trade Ltd based inAnadoluhisari, TURKEY
Concentrated. Peracetic acid-based liquid solution high-level disinfectant with sporicide sterilizing ...
Manufactured by:Bimaks Chemicals Food Foreign Trade Ltd based inAnadoluhisari, TURKEY
Peracetic acid-based highly effective powder disinfectant with sporicide sterilizing ...
Manufactured by:Bimaks Chemicals Food Foreign Trade Ltd based inAnadoluhisari, TURKEY
Combined disinfectant and cleaner for medical devices. Applied by ...