Show results for
Refine by
Surgical Implant Equipment & Supplies In Iraq
98 equipment items found
Manufactured by:LZQ Tool Co., Ltd. based inPudong New District, CHINA
Temporary abutments are designed to form a gingival sulcus and to preserve papillae during the one stage surgical procedure or after a two stage uncovering procedure. Choose an abutment height that is sufficient to laterally support the interproximal papillae without its being too tall such that it would receive undesirable forces from the tongue. Corresponding and matching ...
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
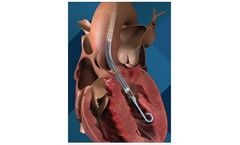
Impella 5.0 is a temporary, minimally invasive heart pump that provides circulatory support, enabling the heart to rest and recover. Impella LD is a surgically implanted heart pump that provides temporary support to the heart during and after a procedure. Impella 5.0 and Impella LD heart pumps allow patients to walk around while on support to improve recovery, if ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Non-bone tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. Non-bone tendons used in ACL reconstruction procedures allow for multiple fixation ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's bone-tendon-bone (BTB) grafts are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These grafts allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Matrix HD Allograft Dermis is an acellular human dermis allograft sterilized to a Sterility Assurance Level (SAL) of 10-6 using the Tutoplast Tissue Sterilization Process. This proprietary process retains the three-dimensional intertwined multidirectional fibers and mechanical properties of the native dermis tissue. The Matrix HD graft provides a natural scaffold to support the body's ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's Achilles tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These tendons allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Conventional allografts are used for various procedures to fill bony voids or gaps in a patient's skeletal system, restore segmental bone loss due to trauma, osteoporosis, removal of tumors or to aid in the surgical correction for a deformity. These grafts provide a scaffold for bone ingrowth to allow for remodeling with the patient’s own bone. These grafts are pre-cut to select sizes to ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical offers the only meniscal allograft with both bone and meniscus sterilized through the BioCleanse® Tissue Sterilization Process. The BioCleanse processed meniscus graft provides a sterilized alternative to traditional aseptically processed meniscus allografts and sets the standard for safety and ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical’s fresh-stored osteochondral (OC) allografts enable surgeons to resurface osteochondral defects with mature hyaline cartilage and repair subchondral bone in a single procedure. Fresh grafts are cleansed, processed and preserved to maintain chondrocyte ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Fortiva Porcine Dermis is a non-crosslinked acellular porcine dermal matrix offering a safe and natural biologic option. Fortiva implants are processed through the Tutoplast® Tissue Sterilization Process and terminally sterilized via validated low-dose gamma irradiation to achieve a sterility assurance level (SAL) of 10-6. Fortiva Porcine Dermis is a ready-to-use biologic implant that acts as ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Cortiva Allograft Dermis is a non-crosslinked acellular dermal matrix offering a safe and natural biologic option and sterilized to a sterility assurance level (SAL) of 10-6 through the Tutoplast® Tissue Sterilization ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Whole bones are used as a sterile source of cortical and cancellous bone. These implants may also be used in procedures that restore segmental and critical bone loss in the extremities due to trauma, bone disease, or from the removal of tumors and/or hardware. After being processed through RTI's BioCleanse® Tissue Sterilization Process, they are terminally sterilized in their final packaging ...
Manufactured by:Corcym UK Limited based inLondon, UNITED KINGDOM
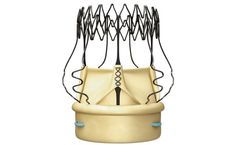
The Perceval Platform is based on a sutureless and collapsible design that simplifies the surgical implantation, reducing the impact of surgery and facilitating faster ...
Manufactured by:KOS LtD based inJUNG-GU, SOUTH KOREA
SPECIFICATIONS: Chemical composition (wt%) : ASTM F2063 - "Standard Specification for Wrought Nickel-Titanium Shape Memory Alloys for Medical Devices And Surgical Implants" ...
Manufactured by:Corcym UK Limited based inLondon, UNITED KINGDOM
Perceval is a pericardial surgical heart valve with a sutureless and collapsible design that simplifies the surgical implantation. Reducing operative trauma and post-operative complications. Enabling faster Patient ...
Manufactured by:R & L Medical based inHangzhou City, CHINA
R&L Medical Calcium Sulphate has obtained NMPA Class III and meets the purity requirements for high-puritycalcium sulfate in surgical implants as specified in ASTM F2224-09, the Standard Specification for High Purity Calcium Sulfate Hemihydrate or Dihydrate for Surgical ...
Manufactured by:Anatomics Pty Ltd. based inMelbourne, AUSTRALIA
Polymer surgical implants are a permanent, predictable and symmetrical option for facial reconstruction or augmentation. StarPore® implants are an advanced polymer tissue scaffold indicated for the repair or augmentation of contours in the skull and face. Made from clinical proven porous polyethylene (HDPE), animal studies have shown ...
Manufactured by:TissX based inMinneapolis, MINNESOTA (USA)
The development of percutaneous cardiac valves (aortic, mitral, tricuspid and pulmonary). The development of surgically implanted cardiac valves. Pericardial Patches and other tissue for surgical, vascular and other applications. Functional components for structural heart disease treatment and other vascular uses. ...
by:Abiomed Heart Recovery based inDanvers, MASSACHUSETTS (USA)
Impella 5.5 with SmartAssist delivers full cardiac support with maximum unloading, allowing the heart to rest and recover. It is a microaxial, surgically implanted heart pump that unloads the left ventricle, reduces ventricular work, and provides the circulatory support necessary to allow recovery and early assessment of residual myocardial function. It is ...
Manufactured by:Axogen (AXGN) based inAlachua, TEXAS (USA)
Axoguard Nerve Protector is the only porcine submucosa extracellular (ECM) matrix surgical implant used to protect injured nerves and to reinforce nerve reconstruction while preventing soft tissue attachments. Designed to protect and isolate, the Axoguard multi-laminar ECM separates and protects the nerve from the surrounding tissues during the healing process. ...