- Home
- Equipment
- north korea
- kidney injury
Show results for
Refine by
Applications
- Artesunate Injection for Malaria
- Artesunate Injection for Malaria Diagnosis of Malaria
- Artesunate Injection for Severe Malaria
- Fluid management solutions for COVID-19 challenging cases sector
- Fluid management solutions for surgery sector
- Fluid management solutions for acute kidney injury (AKI) sector
- Fluid management solutions for enhanced recovery sector
- Fluid management solutions for paediatric sector
Kidney Injury Equipment & Supplies In North Korea
23 equipment items found
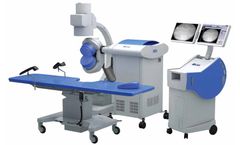
Manufactured by:ACIST Medical Systems based inEden Prairie, MINNESOTA (USA)
CVi delivers the power to improve patient safety, minimizing the risk of contrast-induced acute kidney injury (CI-AKI) for coronary catheterizations when compared to manual injection of contrast ...
Manufactured by:Baxter International Inc. based inDeerfield, ILLINOIS (USA)
The innovative Prismaflex System is designed to support the recovery of critically ill patients with acute kidney injury (AKI). The flexible system meets the demands of multiple therapies with a versatile platform that can be customized to specific patient ...
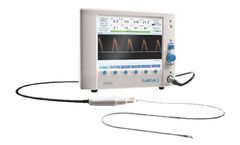
Manufactured by:Terumo Cardiovascular based inAnn Arbor, MICHIGAN (USA)
With the addition of in-line monitoring of oxygen delivery (DO2), CDI® Blood Parameter Monitoring System 550 provides the continuous information needed to reduce the risk of acute kidney injury, improve patient outcomes and save hospital costs. Real-time monitoring of DO2, one of 12 key blood parameters, provides critical information required for goal ...
Manufactured by:Alnylam Pharmaceuticals, Inc. based inCambridge, MASSACHUSETTS (USA)
The first and only FDA-approved prescription medication for the treatment of primary hyperoxaluria type 1 (PH1). OXLUMO is a prescription medicine for the treatment of PH1 to lower oxalate in urine in children and ...
Manufactured by:Links Medical Products Inc. based inIrvine, CALIFORNIA (USA)
The Silent Knight Hazardous Drug (HD) Safety Seal is a simple yet effective new product that is compliant to the USP 800 Standard of safe medication handling to minimize the risk of exposure to healthcare personnel, patients and the environment. The Silent Knight HD Safety Seal works together with the Silent Knight pouch and Silent Knight pill crushing machine to crush, transport and store ...
Manufactured by:InnoSer Belgie NV based inDiepenbeek, BELGIUM
Slow and reproducible cyst formation in the proximal part of the ...
Manufactured by:InnoSer Belgie NV based inDiepenbeek, BELGIUM
Slow progression with cysts in all segments of the ...
Manufactured by:InnoSer Belgie NV based inDiepenbeek, BELGIUM
Quick progression of cyst formation in the distal segment of the ...
Manufactured by:DirexGroup based inCanton, MASSACHUSETTS (USA)
Duet Magna introduces the combination of electromagnetic technology and the double shockwave concept, allowing for some clear and unique advantages in the field of shockwave ...
Manufactured by:Newcells Biotech Limited based inNewcastle Upon Tyne, UNITED KINGDOM
Kidney proximal tubule cell model for safety/efficacy and transporter studies. aProximate™ is one of the most advanced, near physiological, in vitro, kidney proximal tubule cell (PTC) models. aProximate™ PTCs are derived from fresh human kidney tissue and grown on Transwells® where they remain as a functional polarised cell layer that forms tight junctions. In contrast to other ...
Manufactured by:Amivas (US), LLC based inFrederick, MARYLAND (USA)
A Severe Malaria Treatment, Vital for the US Healthcare Community. No FDA-approved injectable malaria medication has been available in the US since IV quinidine was discontinued in early 2019. Artesunate for Injection fills this ...
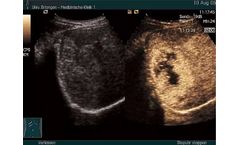
by:Bracco Diagnostic Inc. based inMonroe Township, NEW JERSEY (USA)
Ultrasound is the most widely used and a cost-effective imaging technology. The combination of ultrasound contrast agents with the latest evolving equipment has the potential to transform the clinical role of ultrasound to provide a flexible, cost-effective imaging capability equivalent to existing established modalities such as MRI, CT, and X-ray. ...
Manufactured by:Deltex Medical Limited based inChichester, UNITED KINGDOM
Oesophageal Doppler Monitoring (ODM+) is unique in its ability to guide fluid and drug interventions with accuracy and in real time. The principle is to keep surgical patients in their best haemodynamic state. This means ODM+ helps clinicians to optimise outcomes, with fewer ...
Manufactured by:Newcells Biotech Limited based inNewcastle Upon Tyne, UNITED KINGDOM
Podocyte model for assessing glomerular toxicity. Newcells now offers the first fully differentiated, primary podocyte model derived from fresh kidney tissue for the in vitro assessment of drug-induced glomerular toxicity. The effect of a drug on the glomerular filtration barrier and podocyte glomerular permeability can now be easily evaluated in vitro. Podocyte ...
Manufactured by:Istem Medikal based inSincan, TURKEY
Vesicoureteral reflux (VUR) is the retrograde passage of urine from the bladder into the upper urinary tract. It is the most common urological diagnosis in children, occuring in approximately 1 % of newborns and as high as 30 to 45% in children with UTI. Additionally, there is a high association between VUR, UTI, hypertension and renal damage. ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Covered by Medicare, Prospera is a transplant rejection assessment test that uses a simple blood draw to evaluate the risk of rejection of a transplanted kidney. Through the use of advanced cell-free DNA technology, Prospera increases a provider’s ability to identify otherwise undetected rejection that might lead to kidney loss. Catching transplant rejection as soon as possible can help ...
Manufactured by:DxGen Corp. based inGunpo-si, SOUTH KOREA
Test Kit is for in-vitro diagnostic (IVD) use to determine Glycated Albumin (GA) and Albumin quantitatively from serum or plasma. It is useful at initial assessment, the onset of therapy, and post-therapeutic monitoring of glycemic control. ...
Manufactured by:Kyoto Kagaku Co., Ltd. based inKyoto, JAPAN
A new abdominal phantom to conduct studies and training of fusion imaging for wide range of clinical applications. (CT & ...
Manufactured by:Radiometer Medical ApS based inBrønshøj, DENMARK
Get 19 critical parameters on one tiny blood sample. The ABL90 FLEX PLUS blood gas analyzer is designed for point-of-care testing in busy clinical environments, such as the ED, ICU, NICU or the delivery room where fast results from a very small blood sample are vitally important to make critical diagnostic decisions. The ABL90 FLEX PLUS analyzer gives you reliable results in only 35 seconds on 19 ...
Manufactured by:CTK Biotech, Inc. based inPoway, CALIFORNIA (USA)
The RaFIA Immunofluorescence Analyzer is a fluorescence immunoassay analyzing instrument intended for use by healthcare professionals to aid in the diagnosis of conditions such as cardiovascular disease, pregnancy, infection, diabetes, renal injury and ...