Show results for
Refine by
Hiv 1 Equipment & Supplies In Tuvalu
110 equipment items found
Manufactured by:Atomo Diagnostics Limited based inLeichhardt, AUSTRALIA
Ideally suited for point of care testing, Atomo HIV tests for professional use need only 10uL of capillary blood, and gives an accurate result in just 15 ...
Manufactured by:NOWDiagnostics Inc. based inSpringdale, ARKANSAS (USA)
The ADEXUSDx HIV 1/2 Test is an immunoassay used for the qualitative detection of antibodies to human immunodeficiency virus in whole ...
Manufactured by:Athenese Dx based inChennai, INDIA
The TRUSTline HIV 1/2 Ab Rapid Test is an antibody test for screening blood bank supplies and monitoring HIV prevalence. ...
Manufactured by:Sedia Biosciences Corporation based inBeaverton, OREGON (USA)
A rapid, visually read, in vitro immunoassay for the qualitative detection of antibodies to HIV-1 and HIV-2 in oral fluid specimens. Will be intended for use outside the United States. ...
Manufactured by:Sedia Biosciences Corporation based inBeaverton, OREGON (USA)
An in vitro single well quantitative limiting antigen avidity enzyme immunoassay for distinguishing recent HIV-1 infections from those which are long-term. Intended for use with liquid serum or plasma specimens. ...
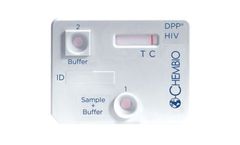
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
An FDA approved, rapid point-of-care test that detects antibodies to HIV-1 and HIV-2. ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
MaximBio's Cambridge Biotech HIV-1 Urine Western Blot Kit is an FDA approved in-vitro qualitative assay for the detection and identification of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) contained in human urine. This more specific assay is used as a supplemental test with urine specimens that ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
Maxim's FDA Approved HIV-1 Urine EIA test is an enzyme immunoassay for the in-vitro detection of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) in urine. The test is intended for use in professional laboratory settings as an aid in clinical diagnosis of HIV infection. Before a ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
The Maxim HIV-1 Limiting Antigen-Avidity EIA is an in-vitro quantitative limiting antigen (LAg) avidity enzyme immunoassay for distinguishing recent HIV-1 infections from those which are long-term. Persons with recently acquired HIV-1 infections typically exhibit ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
The Maxim HIV-1 Limiting Antigen-Avidity Dried Blood Spot (DBS) EIA is an in-vitro quantitative limiting antigen (LAg) avidity enzyme immunoassay for distinguishing recent HIV-1 infections from those which are long-term. Persons with recently acquired HIV-1 infections typically exhibit ...
Manufactured by:Atomo Diagnostics Limited based inLeichhardt, AUSTRALIA
The only HIV Self Test listed on the Australian Register of Therapeutic Goods ...
Manufactured by:Linear Chemicals S.L.U. based inMontgat, SPAIN
A rapid one step test for the qualitative detection of antibodies (IgG, IgM, IgA) to anti-HIV-1(including O) and-2 virus and HIV-1 p24 antigen in human serum, plasma, whole ...
Manufactured by:Creative Peptides based inNew York, NEW YORK (USA)
Enfuvirtide is a 36 amino acid peptide corresponding to a region of gp41, the transmembrane subunit of HIV-1 envelope protein. It belongs to the therapeutic class of fusion inhibitors and acts by binding to gp41 and impeding the conformational changes in gp41 necessary for fusion of the virus with the ...
Manufactured by:Bio-Rad Laboratories, Inc. based inHercules, CALIFORNIA (USA)
The BioPlex 2200 HIV Ag-Ab assay is a multiplex flow immunoassay intended for the simultaneous qualitative detection and differentiation of the individual analytes HIV-1 p24 antigen, HIV-1 (groups M and O) antibodies, and HIV-2 antibodies in human serum or plasma (fresh or frozen K2 EDTA, K3 ...
Manufactured by:Cone Bioproducts based inSeguin, TEXAS (USA)
Individual donor units used in the preparation of this product have been tested and found to be negative or non-reactive for HBsAg, Anti-HIV I/II, Anti-HCV, HIV-1 RNA, HCV RNA. Donors were tested and found negative for Syphilis according to FDA ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
The Maxim Swift HIV Recent Infection Assay (RIA) is a single use qualitative immunoassay to detect the circulating antibodies to Human Immunodeficiency Virus Type 1 (HIV-1), Type 2 (HIV-2) and distinguish between recent and long-term infection in HIV-1/2. The assay is a point of ...
Manufactured by:Chembio Diagnostic Systems, Inc. based inHauppauge, NEW YORK (USA)
An FDA Approved, dual rapid test for the detection of antibodies to HIV 1/2 and Treponema pallidum in fingerstick whole blood, venous whole blood, or plasma specimens. ...
Manufactured by:Frontier Biotechnologies Inc. based inNanjing, CHINA
To develop an all-injectable long-acting HIV therapy, Frontier Biotech has designed and developed a combination regimen of Albuvirtide (ABT) and 3BNC117, a recombinant fully human monoclonal antibody with broadly neutralizing effects to HIV-1 ...
Manufactured by:Access Bio based inSomerset, NEW JERSEY (USA)
The CareStart™ EZ HIV 1/2 is an in vitro RDT for the qualitative detection of antibodies to HIV type-1 and/or type-2 in serum, plasma, capillary whole blood or venous whole blood. The device is a single-use intended for healthcare professional use as an aid to diagnosis only – not for screening blood ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Efavirenz (DMP 266) is a potent inhibitor of the wild-type HIV-1 reverse transcriptase with a Ki of 2.93 nM and exhibits an IC95 of 1.5 nM for the inhibition of HIV-1 replicative spread in cell ...