Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Aortic Stented Tissue Valve Equipment & Supplies In Usa
56 equipment items found
Manufactured by:Abbott based inSanta Clara, ALABAMA (USA)
The Epic™ Max Aortic Stented Tissue Valve represents the latest advancement in aortic valve replacement technology, engineered to optimize clinical outcomes and enhance lifetime patient management. It features porcine leaflets for natural leaflet functionality, minimizing calcification and ...
Manufactured by:Abbott based inSanta Clara, ALABAMA (USA)
The Epic Plus Mitral and Aortic Stented Tissue Valves from Abbott represent the latest generation of the Epic Platform, designed for patients requiring heart valve replacement due to diseased, damaged, or malfunctioning valves. These bioprosthetic valves are engineered with ...
Manufactured by:Genesee BioMedical, Inc. based inDenver, COLORADO (USA)
Dual Design for Multiple Approaches AVATR is an aortic valve and thoracic retractor for MICS procedures. Left thoracotomy and redo… ...
Manufactured by:Evaheart, Inc. (EVI) based inHouston, TEXAS (USA)
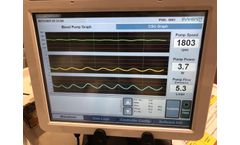
The EVAHEART 2 External Monitor System is a user-friendly and easily adaptable interface for display of device-related parameters for clinician use. Real-time waveforms for blood pump parameters and flow ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible ...
Manufactured by:Evaheart, Inc. (EVI) based inHouston, TEXAS (USA)
The Hydraulically levitated impeller uses an ultra-thin layer of circulating sterile water which lubricates the mechanical seal interface and cools the area around the shaft to minimize the risk of clot formation. The Open-vane impeller with large flow gaps allow for excellent blood washout and operation at lower speeds to reduce blood sheer. Pump Speed Modulation allows for customizable speed ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
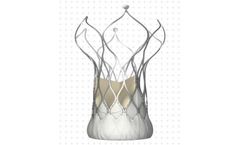
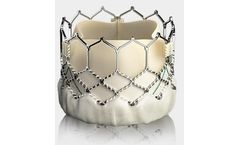
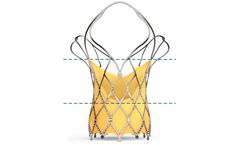
Built on the proven SAPIEN platform, the SAPIEN 3 Ultra transcatheter heart valve was designed with patient needs in ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Designed for predictable deployment. The SAPIEN XT valve may be used in the aortic position via a broad range of access routes - transfemoral, transapical and ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X Ascending Aortic Prosthesis with the Vascutek Gelweave Valsalva Graft. The On-X Ascending Aortic Prosthesis with the Vascutek Gelweave Valsalva Graft is the only valve/graft combination to offer: A generous PTFE sewing ring for ease of suturing; The first coated valsalva graft for optimal coronary ostia ...
Manufactured by:Vscan Rocks, by GE HealthCare based inSuzhou, CHINA
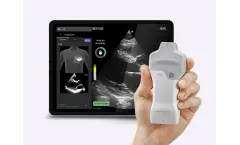
Providing rapid echocardiographic assessments at the point of care can be difficult with time and resource constraints. Caption AI helps you overcome those challenges. With this powerful and easy-to-use tool, you can bring focused echo to any patient anytime – and get them on the right care path ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible ...
Manufactured by:Anteris Technologies Limited based inToowong, AUSTRALIA
The World’s First & Only 3D Single-piece Aortic Valve. DurAVR™ heart valve has the potential to deliver a functional cure in the treatment of Severe Aortic Stenosis. The unique design of DurAVR™ coupled with the ADAPT® technology provide the potential to deliver a more durable heart valve that delivers better hemodynamics ...
Manufactured by:MicroPort Scientific Corporation based inShanghai, CHINA
VitaFlow Aortic Valve, uses bovine pericardial leaflets to provide enhanced valve durability, its innovative frame and skirt designs deliver reliable hemodynamic performance, and dependable clinical outcomes for ...
Manufactured by:Edwards Lifesciences Corporation based inIrvine, CALIFORNIA (USA)
Builds upon the proven strengths of the SAPIEN valve with 5 years of durability. The SAPIEN 3 valve is the only valve approved for valve-in-valve procedures in both the aortic and mitral positions, allowing patients at high or greater surgical risk to avoid an additional open heart ...
Manufactured by:Biosensors International Group, Ltd. based inSingapore, SINGAPORE
The ALLEGRA Transcatheter Heart Valve integrated with the IMPERIA Delivery System offers a state-of-the-art solution for transcatheter aortic valve interventions. Designed to maintain hemodynamic stability throughout deployment, the Permaflow Delivery System allows for continuous blood flow, providing medical professionals with the time needed to accurately position the device without stress. The ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
The On-X Aortic Valve is a newer generation heart valve made of a unique material and design characteristics compared with earlier generations of mechanical heart valves. The On-X Aortic Valve is the only mechanical valve with FDA and CE approval to be used safely with less blood thinner (warfarin). The AHA and ACC guidelines state that less blood thinner may be reasonable for patients with the ...
Manufactured by:Sahajanand Medical Technologies Ltd. based inSurat, INDIA
Self-expanding TAVI device to have 2 rows of marker; First Markers are located at Node 1. Second Markers are located at Node 3. First marker helps; In precise implantation of the valve at the targeted implantation zone. To ascertain the depth of implant. Second marker indicates; When the THV leaflets are going to get ...