Refine by
Approved Peptide Drugs Equipment & Supplies
125 equipment items found
Manufactured by:Beldimed s.a. based inAndrimont, BELGIUM
The expression “Patient First” is part of our DNA. We truly believe that every person in the world should have the same opportunities when it comes to healthcare because health is the ...
by:Curemark based inRye Brook, NEW YORK (USA)
More than 5 million children in the U.S., aged 4-17 years old, have been diagnosed with Attention-Deficit Hyperactivity Disorder (ADHD). The condition is characterized by inattention, hyperactivity and impulsivity. Current prescription medications and psychosocial therapies focus on ameliorating primary symptoms. No currently approved drug treats the underlying cause of ADHD, and there is no ...
Manufactured by:Creative Peptides based inNew York, NEW YORK (USA)
Dalbavancin (INN, trade name Dalvance) is a novel second-generation lipoglycopeptide antibiotic. It belongs to the same class as vancomycin.The Food and Drug Administration approved dalbavancin for the treatment of acute bacterial skin and skin structure infections caused by certain susceptible bacteria.https://www.creative-peptides.com/product/dalbavancin-item-10-101-78-32.html ...
Manufactured by:RareStone Group based inShanghai, CHINA
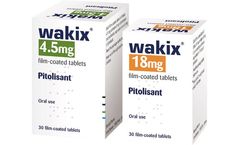
WAKIX® (pitolisant) is a narcolepsy treatment that Citrine has in-licensed from the French biotech company, Bioprojet, for use in China. Pitolisant is a selective histamine H3-receptor antagonist/inverse agonist, which increases the release of the brain chemical histamine to increase a patient's wakefulness and alertness. The drug was approved by the European Medicines Agency (EMA) in 2016 ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
Post-exposure prophylaxis (PEP) regimens are designed to block or reduce impact of infections in individuals who have known or potential exposure to an infectious agent but are not symptomatic. Such regimens have been used successfully for other infections. In October 2018, SIGA entered into a Cooperative Research and Development Agreement (CRADA) with the United States Army Medical Research ...
Manufactured by:Eleven P15 based inDenver, COLORADO (USA)
Idiopathic Pulmonary Fibrosis (IPF) is a chronic, fibrosing interstitial pneumonia of unknown cause, occurring primarily in older adults, and localized to the lung. IPF is a fatal disease with median survival at 3-5 years. Two drugs have been approved for treatment of IPF, but these drugs fail to reverse scarred lung tissue or to improve survival. Accurate diagnosis of IPF is ...
by:Starton Therapeutics based inParamus, NEW JERSEY (USA)
STAR-LLD is in development in two continuous delivery systems: subcutaneous and transdermal. STAR-LLD is being developed to establish the only IMiD (immunomodulatory drug) approved for CLL. CLL is the most common form of leukemia. Lenalidomide has been shown to be efficacious and well tolerated in CLL previous randomized controlled trials did not obtain an indication for the product. Even in the ...
Manufactured by:Amivas (US), LLC based inFrederick, MARYLAND (USA)
Artesunate for Injection is an antimalarial indicated for the initial treatment of severe malaria in adults and ...
Manufactured by:EndoLogic LLC based inClifton, NEW JERSEY (USA)
Renzapride is best suited as a treatment of Diabetic Gastroparesis, a condition with a significant unmet treatment options. In the numerous trials that have been done using Renzapride more than 5000 patients have been treated with Renzapride. An extensive cardiac safety evaluation under the FDA mandated Thorough QT trial has shown that Renzapride does not have any cardiac safety ...
by:Genmab A/S based inCopenhagen V, DENMARK
Daratumumab is a human IgG1k monoclonal antibody (mAb) that binds with high affinity to the CD38 molecule, which is highly expressed on the surface of multiple myeloma cells.1 Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license to develop, manufacture and commercialize daratumumab from Genmab. A subcutaneous formulation of daratumumab, DARZALEX ...
Manufactured by:Inoviem Scientific SAS based inIllkirch-Graffenstaden, FRANCE
Identify the mode of action of your compound with NPOT® technology; NPOT technology is a unique heterogeneous assembly method for conducting extensive identification of all proteins (together with their native structure) involved in drug-target ...
Manufactured by:BioQ Pharma based inSan Francisco, CALIFORNIA (USA)
invenious connect-and-go technology is already being used to deliver infusibles in a patented range of infusion systems. Our products are thoughtfully-designed to each drug – with bespoke solutions for: Drugs requiring complex weight-based dosing. Cytotoxic drugs. Drugs that need to be reconstituted or diluted at time of use. Drug-drug combinations, including for cold-chain ...
Manufactured by:SIGA Technologies, Inc. based inNew York, NEW YORK (USA)
Oral TPOXX® (tecovirimat) is the first drug approved by the U.S. Food and Drug Administration (FDA) that is specifically indicated for the treatment of smallpox disease in adults and pediatric patients weighing at least 13 kg. (Prescription Label) TPOXX® inhibits viral maturation of variola virus (the virus that causes smallpox) and other poxviruses by preventing the formation of a ...
Manufactured by:Cellworks Research India Private Limited based inBangalore, INDIA
The Cellworks Platform biosimulates how a patient’s unique cancer will respond to therapies prior to treatment and identifies novel drug combinations for treatment-refractory patients. Therapy biosimulation is the ability to predict the phenotype response of a human cell to an external stimulus, such as a drug ligand or radiation. We utilize this technology, to determine a patient’s ...
Manufactured by:Auro Vaccines LLC based inPearl River, NEW YORK (USA)
Auro Vaccines has developed VesiculoVax™-vectored vaccines for pre- and post-exposure protection against the hemorrhagic disease caused by multiple species of filovirus (i.e. Zaire ebolavirus, Sudan ebolavirus and Marburg ...
Manufactured by:Quanterix Corporation based inBillerica, MASSACHUSETTS (USA)
Unleashing the power of next-generation Simoa® Planar Array technology for robust multiplex circulating biomarker detection at the earliest stages of disease progression - even at healthy baseline levels. The Quanterix SP-X Imaging and Analysis System is a complete benchtop system that offers true multiplex detection at both acute and baseline levels. For the first time, oncology and ...
Manufactured by:UroGen Pharma, Inc. based inPrinceton, NEW YORK (USA)
UroGen is developing UGN-102 (mitomycin) for intravesical solution as a primary nonsurgical alternative to repetitive TURBT. UGN-102 is an investigational formulation that utilizes our innovative technology, RTGel™ reverse-thermal hydrogel, for the treatment of low-grade NMIBC. Instilled via standard catheters, UGN-102 is designed to dwell for a period of several hours before being excreted ...
Manufactured by:KORU Medical Systems based inMahwah, NEW JERSEY (USA)
With the performance of the familiar FREEDOM60® in an even simpler design for 20ml and 30ml syringes comes the FreedomEdge® – a compact infusion solution that’s easy to use for smaller or often-repeated doses. It is so simple it makes frequent and flexible dosing a reality – in a safe, controlled manner. Just close and go. ...
Manufactured by:Catalent, Inc based inSomerset, NEW JERSEY (USA)
GPEx cell line development technology is a proven technology that generates high-performance, highly stable, production cell lines. Virtually any cDNA (mAb and multigenic applications) can be inserted into cells. To date, over 600 different mAb and mAb fusions and over 160+ different recombinant proteins have been produced using the GPEx system, with 160+ clinical trials utilizing therapeutic ...
Manufactured by:Vericel Corporation based inCambridge, MASSACHUSETTS (USA)
The U.S. Food and Drug Administration has approved MACI (autologous cultured chondrocytes on porcine collagen membrane) for the repair of symptomatic, full-thickness cartilage defects of the knee in adult patients. MACI is the first FDA-approved product that applies the process of tissue engineering to grow cells on scaffolds using healthy cartilage tissue from the patient’s own knee. ...