- Home
- Equipment
- usa california
- biomark
Show results for
Refine by
Biomark Equipment Supplied In Usa California
63 equipment items found
Manufactured by:bioMérieux S.A. based inMarcy l`Etoile, FRANCE
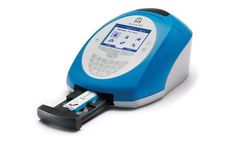
The Astute140 Meter is a bench-top/tabletop analyzer that converts the fluorescent signal from each of the two biomarker assays, TIMP-2 and IGFBP-7, contained within the Test Cartridge into a single numerical result. The NephroCheck Test results are available on the Astute140® Meter in approximately 20 ...
Manufactured by:Proxim Diagnostics Corp. based inSanta Clara, CALIFORNIA (USA)
Cardiac Troponin is a heart attack blood biomarker that is measured in patients who present symptoms such as chest pain upon arrival in an emergency department. Compared to conventional Troponin tests, our high-sensitivity Troponin I (hs-cTnI) assay enables earlier detection and reliable rule-out. ...
Manufactured by:Biocept based inSan Diego, CALIFORNIA (USA)
Combining Biocept’s Liquid Biopsy biomarker testing expertise with Oncomine™ from Thermo Fisher Scientific’s next-generation sequencing and decision support ...
Manufactured by:Biocept based inSan Diego, CALIFORNIA (USA)
Combining Biocept’s Liquid Biopsy biomarker testing expertise with Oncomine™ from Thermo Fisher Scientific’s next-generation sequencing and decision support ...
Distributed by:UCP Biosciences Inc. based inSan Jose, CALIFORNIA (USA)
This kit is used for the in vitro quantitative detection of Cardiac Troponin I (cTnI), Creatine Kinase MB (CK-MB), Myoglobin (MYO) concentration in human serum/plasma and whole blood samples. Cardiac troponin I: cardiac troponin I (cTnI) is a subunit of the cardiac troponin complex that regulates actin and myosin interactions by regulating the activity of Ca2+ on rhabdomyosin atpase. When ...
Distributed by:UCP Biosciences Inc. based inSan Jose, CALIFORNIA (USA)
This kit is used for the in vitro quantitative determination of Cardial Troponin (cTnI) concentration in human serum/plasma or whole blood samples. Cardiac troponin I(cTnI), a 24kDa subunit of the troponin complex composed of 209 amino acids, regulates actin and myosin interactions by regulating the activity of Ca2+ on rhabdomyosin atpase.After myocardial injury, the cTnI complex was released ...
Distributed by:UCP Biosciences Inc. based inSan Jose, CALIFORNIA (USA)
This kit is used for the in vitro quantitative detection of D-Dimer concentration in human plasma and whole blood samples. D-Dimer is a specific degradation product produced by fibrin monomer crosslinked with activation factor and hydrolyzed by plasmin. Its formation and elevation reflect the activation of coagulation and fibrinolytic system of the body and are specific indicators of ...
Manufactured by:CTK Biotech, Inc. based inPoway, CALIFORNIA (USA)
The Positivia FOB Rapid Test Control Kit is intended for use with the OnSite FOB and FOB-Hi Rapid Tests as external assay controls to monitor test ...
Manufactured by:Trukera Medical, formely known as TearLab based inEscondido, CALIFORNIA (USA)
TearLab® Osmolarity System - An Essential Diagnostic for Dry Eye Disease. TearLab is an objective and quantitative point-of-care diagnostic test that provides precise and predictive information. The TearLab Osmolarity System* is intended to measure the osmolarity of human tears to aid in the diagnosis of dry eye disease in patients suspected of having dry eye disease, in conjunction with ...
by:Ionpath, Inc. based inMenlo Park, CALIFORNIA (USA)
Specially formulated high-purity gold-coated slides let your biomarker signals shine without common slide interferences, such as chromium or titanium. Qty ...
Manufactured by:DxTerity Diagnostics Inc. based inRancho Dominguez, CALIFORNIA (USA)
SLE Monitor combines three widely used biomarkers of SLE disease activity with the DxTerity IFN-1 test. The SLE Monitor Test is undergoing evaluation as a "from home" test. Check back for information about enrolling in the ELEVATE clinical ...
Manufactured by:Kura Oncology, Inc. based inSan Diego, CALIFORNIA (USA)
This is a major advance over traditional chemotherapy, which can be as potent against healthy tissue as it is against cancer. Using tumor genomic profiling and biomarker strategies, physicians can select the patients most likely to respond well to targeted therapies. Yet, so far, due to exquisite selectivity of these new precision medicines, only relatively smaller patient ...
Manufactured by:bioMérieux S.A. based inMarcy l`Etoile, FRANCE
The NephroCheck Test Kit is a single-use cartridge designed to detect biomarkers of acute kidney injury, TIMP-2 and IGFBP-7. The test provides results in 20 ...
Distributed by:Rockley Photonics Ltd based inPasadena, CALIFORNIA (USA)
A Holistic Approach to Wearable Health Monitoring. Biosensing for Professional Healthcare: Leveraging the unique biosensing capabilities that Rockley’s photonics-based technology enables, the Bioptx product line will address the specific needs of the professional healthcare market — including hospitals, research clinics, pharmaceutical companies, medical device manufacturers, ...
Manufactured by:OptraSCAN Inc. based inSan Jose, CALIFORNIA (USA)
The scanner automatically identifies regions to scan and simultaneously analyzes the tissue/cell area being scanned based on tissue morphology and type of biomarker. This eventually benefits the end user to view the whole slide scanned image along with analyzed output as overlay during their review process. This analyzed information thus act as reference Heatmap guiding the users ...
Manufactured by:Biocept based inSan Diego, CALIFORNIA (USA)
his kit detects BRAF V600 mutations in DNA derived from blood plasma or FFPE tissue sections to give insight into cancer characteristics and provide Biomarker status of tumors such as ...
Manufactured by:Biocept based inSan Diego, CALIFORNIA (USA)
This kit detects EGFR mutations in DNA derived from blood plasma or FFPE tissue sections to give insight into cancer characteristics and provide Biomarker status of tumors such as non-small cell lung ...
Manufactured by:Krishgen Biosystems based inMumbai, INDIA
Validated against seven points for a Gold Ring Standard Quality ELISA - the benchmark sign for Krishgen quality, Long shelf-life, Expert technical support. The GENLISA ELISA kits are used for assessing the specific biomarker in samples analytes which may be serum, plasma, biological fluids and cell culture supernatant. The kit employs a double-antibody sandwich ELISA technique ...
by:Natera, Inc. based inAustin, TEXAS (USA)
Support treatment decisions with comprehensive tumor profiling. Gain deep insight into the tumor’s genomic alterations and oncology biomarkers with Altera. The detailed sequencing report includes FDA-approved treatments, novel treatment approaches and ongoing clinical trials targeting found mutations. Unlike any other tumor profiling assay, Altera also enables molecular ...
Manufactured by:Olympia Diagnostics, Inc. based inSunnyvale, CALIFORNIA (USA)
A prognostic test kit quantitatively measures the expression levels of a panel of cancer recurrence-associated biomarkers in patient urine sample or biopsy tissue sample to predict cancer recurrence and patient ...