Refine by
Biosafety Equipment & Supplies
56 equipment items found
by:PatoGen Analyse AS based inAalesund, NORWAY
PatoGen BioSafety Program™ is a comprehensive system for minimising the risk of disease and loss throughout the production cycle. PatoGen's aim is for the program both to prevent the introduction of infection as a result of a lack of knowledge of infection status in individual populations and enable potential disease problems to be ...
Manufactured by:Regenhu based inVillaz-St-Pierre, SWITZERLAND
The 3d bioprinting station in a biosafety enclosure: bringing the future of tissue engineering and regenerative medicine closer by revolutionizing medicine ...
Manufactured by:Advanced Solutions Life Sciences based inLouisville, KENTUCKY (USA)
The six-axis robotic arm and the collection of tools make BAB500 a unique offering for tissue engineering. With it being housed in a biosafety cabinet to ensure a sterile environment, life scientists will have further control over all parameters in their ...
Manufactured by:Advanced Solutions Life Sciences based inLouisville, KENTUCKY (USA)
BioAssemblyBot 200 is built on the foundation of 50,000+ hours of research and development that created the BioAssembly platform. BAB200 takes three forms: biosafety cabinet ready, enclosed, and enclosed with HEPA filtration. Using a four-axis arm to bioprint, pick up well plates, and pipette material, BAB200 is built for the modern ...
Manufactured by:MR Solutions based inGuildford, UNITED KINGDOM
Utilizing proprietary Dry Magnet technology, this system does not require liquid helium or nitrogen for cooling, making installation achievable even in biosafety labs with minimal site preparation. Its compact design weighs merely 700 kg compared to conventional wet magnets which can weigh up to 5 tons, easing the logistics of setting up advanced research facilities. Notably, the ...
Manufactured by:Aseptic Technologies based inGembloux, BELGIUM
This system consists of a filling unit, a dosing system, precision balance, laser re-sealing unit, and a capping tool, all capable of being installed in a biosafety cabinet or isolator. Offering flexibility, the Crystal M1 can fill up to 200 AT-Closed Vials and supports volumes ranging from 0.1 ml to 50 ml. This system is particularly suited for small and personalized batch ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
Testing for Ebola Virus Disease (EVD) must be performed in accordance with current guidelines provided by the appropriate public health authorities that address appropriate biosafety conditions, interpretation of test results, and coordination of testing, results and patient management with public health authorities. ...
Manufactured by:OraSure Technologies based inBethlehem, PENNSYLVANIA (USA)
Testing for Ebola Virus Disease (EVD) must be performed in accordance with current guidelines provided by the appropriate public health authorities that address appropriate biosafety conditions, interpretation of test results, and coordination of testing, results and patient management with public health ...
Manufactured by:Scie-Con Scientific Consulting LLC based in, USA
The GeSiM BioScaffolder is a customizable bioprinting multitool for regenerative medicine, tissue engineering and biomaterials research and it fits into most biosafety cabinets. It offers a variety of tools used for tissue engineering, scaffolding of bone and cartilage as well as soft tissues, electroactive tissues, heart valves and vasculature. It has applications in 3D printing ...
Manufactured by:Gloveboxes Group based inAmesbury, MASSACHUSETTS (USA)
They also integrate advanced equipment like bio-decontamination units and rapid transfer ports. Gloveboxes Group's solutions include tailor-made laminar-flow biosafety cabinets, weighing booths, and transfer trolleys, ideal for applications like nanoparticle cancer drugs and surgical implants. These isolators provide a controlled environment that minimizes cross-contamination and ...
Manufactured by:Gloveboxes Group based inAmesbury, MASSACHUSETTS (USA)
With integrated solutions such as bio-decontamination ports and stopper machines, Gloveboxes Group offers advanced systems that protect APIs, HPAPIs, and operators. Their expertise extends to restricted access barrier systems (RABS), biosafety cabinets, and custom transfer trolleys. Leveraging automation technology, they ensure efficient, safe, and energy-optimized operations, ...
Manufactured by:PP-L Biosafety based inSleaford, UNITED KINGDOM
The IDEAL - H14 Medical Grade HEPA Filter is engineered to provide efficient air cleaning solutions particularly suited for environments with high demands for air purity. Manufactured in Germany, this device offers a clean air flow rate of up to 810 cubic meters per hour, making it a reliable choice for mitigating airborne virus transmission risks such as those posed by coronaviruses. Its ...
Manufactured by:Cellomics Technology, LLC based inHalethorpe, MARYLAND (USA)
C2C12 cells have been widely used in myoblast differentiation research. C2C12/Cre-GFP stable cells express Cre recombinase fused with GFP under the control of the CMV promoter. Cre-loxP system has been widely used for gene editing such as deletion, inversion and translocation. This Cre-GFP stable cell line is convenient for using to test your loxP constructs in C2C12 ...
by:OSSIO Inc based inWoburn, MASSACHUSETTS (USA)
Safety Unmatched: Bio-Integrative OSSIOfiber - A first-of-its-kind material technology that delivers peace of mind through predictable implant integration without encapsulation or adverse ...
Manufactured by:OriGene Technologies, Inc. based inRockville, MARYLAND (USA)
OriGene offers pre-made lentiviral shRNA plasmid kits containing 4 sequence-verified expression cassettes that target your gene of interest. Each lentiviral cassette contains three major functional elements: shRNA, a puromycin selection marker & a tGFP reporter. With a guaranteed knockdown of ≥70%, these kits and our ready-to-use lenti-shRNA particles are a great option for studying ...
Manufactured by:Cellomics Technology, LLC based inHalethorpe, MARYLAND (USA)
Lentiviral vector-CAG, BSD contains Multiple Cloning Sites (MCS) downstream of the CAG promoter, and blasticidin driven by the PGK promoter as a selection marker. Clone your gene of interest into the MCS, then co-transfect with EZ-LentiPACK Packaging System into packaging cells, such as HEK293T to produce VSV-G pseudotyped lentivirus. Transduced stable cells can be selected by ...
Manufactured by:Tecres S.p.A. based inSommacampagna (VR), ITALY
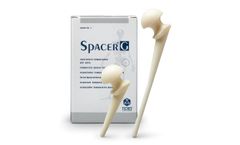
Spacer®-G is the preformed spacer for hip preloaded with Gentamicin. The device includes a load-bearing structure in stainless steel, with a standard tapered stem. It’s available in 3 different head sizes (46-54-60mm) and 2 different stem ...
Manufactured by:Tecres S.p.A. based inSommacampagna (VR), ITALY
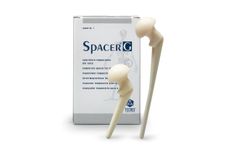
Spacer®-G Flat Stem is the preformed spacer for hip preloaded with Gentamicin. The device includes a load-bearing structure in stainless steel, with a rectangular flat stem. It’s available in 3 different head sizes (46-54-60mm) and 2 different stem ...
Manufactured by:Xingtai Jiachuang Technology Co., Ltd. based inXingtai City, CHINA
High purity HGH CAS 9002-72-6 ex-factory price 99%. Main functions: Promote growth, Cell regeneration, Metabolism. Color: White powder. Customize: receive. Specification: ...
Manufactured by:Anthogyr - Institut Straumann AG based inSallanches, FRANCE
Axiom® Tissue Level implant promotes biological safety and easy prosthesis insertion at the gingival level. It helps to preserve the epithelium and connective tissue attachment. The Axiom® TL implant is available in the REG profile, suitable for most clinical indications, and PX profile preferred for immediate post-extraction implantation for better bone anchorage. Axiom® TL ...