- Home
- Equipment
- north america
- blood stem cell donation
Refine by
Blood Stem Cell Donation Equipment Supplied In North America
6 equipment items found
Manufactured by:Verax Biomedical, Incorporated based inMarlborough, MASSACHUSETTS (USA)
Verax is currently developing a version of its PGD test to address the risk of bacterial contamination in red blood cells. While the risk of red blood cell contamination is lower than the risk of platelet contamination, at 1:20,000, it is the second largest infectious risk for the nation’s blood supply and far exceeds the risk associated with transfusion-transmitted viruses such as HIV, ...
Manufactured by:HnG Medical Incorporated based inNorth York, ONTARIO (CANADA)
Accommodating the hand-held sealing head (Model 1105), the system adjusts automatically for different sizes of tubing. The durable design fits in virtually any work area, and is ideally suited for mobile whole blood donation areas, component processing centers and stem cell laboratories. SEBRA sealers are based on ...
Manufactured by:Hope Biosciences based inSugar Land, TEXAS (USA)
The truth is that almost everyone that has banked their newborn's stem cells has banked for the purpose of banking rather than for the purpose of using. According to the American Society for Blood and Marrow Transplantation (ASBMT), the chance of a baby later benefiting his or her own banked cord blood is currently less than 0.04 percent. Hope Biosciences, is first and foremost, a stem cell ...
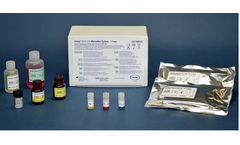
Manufactured by:Avioq, Inc. based inResearch Triangle Park, NORTH CAROLINA (USA)
The Avioq HTLV-I/II Microelisa System is intended as a screen for donated blood and organs to prevent transmission of HTLV-I and HTLV-II to recipients of cellular blood components and as an aid in clinical diagnosis of HTLV-I or HTLV-II infection and related ...
Manufactured by:Premier Biotech, Inc. based inMinneapolis, MINNESOTA (USA)
The Premier Biotech COVID-19 IgG/IgM Rapid Test Cassette is a lateral flow immunochromatographic assay for the detection of SARS-CoV-2 antibodies in venous whole blood, serum or plasma. The COVID-19 IgG/IgM Rapid Test Cassette is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. COVID-19 (Coronavirus ...
Manufactured by:Able Diagnostics, Inc. based inSan Diego, CALIFORNIA (USA)
The 1st Point-of-Care (POC) Antibody Test for COVID-19 with Fingerstick Whole Blood Authorized by FDA!. Click the link to review FDA NEWS RELEASE on Sept 23, 2020 in details: "FDA Authorizes First Point-of-Care Antibody Test for COVID-19" about Assure COVID-19 IgG/IgM Rapid Test Device. This authorization means that Fingerstick Blood Samples can now be tested in POC settings like Doctor's ...