Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Applications
- Clinical Immunology and Intensive Care Medicine for Haemophilia
- Clinical Immunology and Intensive Care Medicine for Immunoglobulins
- Clinical Immunology and Intensive Care Medicine for Antibody Deficiency
- Drug Dolution for Liver Metastases
- Drug Dolution for Gastric Cancer
- The mat (t) isse bioprothesis: a bio-resorbable tec (tissue engineering chamber)
- Nuclear Medicine for Therapy
- Nuclear Medicine for Theragnostics
Clinical Data On Brain Development Equipment Supplied In Europe
88 equipment items found
Manufactured by:HepaRegeniX GmbH based inTübingen, GERMANY
HepaRegeniX has started to progress the clinical development of its proprietary candidates e.g. ...
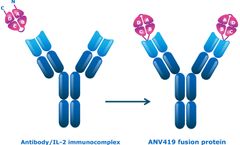
Manufactured by:Anaveon AG based inBasel, SWITZERLAND
The culmination of our research is ANV419, which is in clinical development. It is a rationally engineered fusion protein that is a stable, antibody-like molecule. ...
Manufactured by:Aristotech Industries GmbH based inLuckenwalde, GERMANY
The Design of POROCUP is based on the principles of the well-known and long term clinically proven TITANIUM CUP developed in Switzerland in the 90´s. Applying a Titanium Forging Technology, a macro porosity surface is created, which favors the bone osteointegration without the necessity of additional ...
Manufactured by:Aristotech Industries GmbH based inLuckenwalde, GERMANY
Femoral Stem Forgings based on the design concepts of clinically proven uncemented stems, developed in the ...
Manufactured by:Genenta Science based inMilano, ITALY
After apheresis collection, a functional copy of the therapeutic gene is inserted into the patient’s own HSPCs using a non-replicating lentiviral vector. This is an ex-vivo process called transduction. We believe that LVVs are the first choice for ex-vivo gene therapy in humans because they can efficiently transduce HSPCs with potentially large transgenes that will allow us to expand ...
Manufactured by:Interacoustics based inMiddelfart, DENMARK
A versatile device offering auditory evoked potentials (AEP), auditory steady state response (ASSR), vestibular evoked myogenic potentials (VEMP) and otoacoustic emissions (OAE). Designed to fit seamlessly into your workflow and offers complete reliability and perfect results. ...
by:STALICLA based inGeneve, SWITZERLAND
DDU validates ASD phenotype groups and drug candidates through observational studies and investigational trials. DDU combines expertise in operations, medicine, drug and clinical development, to bring STALICLA’s clinical-stage treatment options closer to ...
Manufactured by:BioArctic AB based inStockholm, SWEDEN
The drug candidate ABBV-0805 is a monoclonal antibody that selectively binds and eliminates oligomers and protofibrils of alpha-synuclein. The goal is to develop a disease modifying treatment that stops or slows down disease ...
Manufactured by:Illumina Inc. based inSan Diego, CALIFORNIA (USA)
The NextSeq 550Dx instrument is FDA regulated and CE-in vitrodiagnostic (IVD) marked, enabling clinical laboratories to develop and perform a wide range of applications, from NGS IVD assays using targeted panels, to clinical research applications that include methods from targeted panels to whole ...
Manufactured by:Biotest AG based inDreieich, GERMANY
At the ISTH 2020 Biotest presented its novel recombinant Factor VIII molecules overcoming the major challenges of current Factor VIII replacement therapy by combining a significantly extended half-life, low immunogenicity and the possibility of either subcutaneous or intravenous ...
Manufactured by:Vivesto AB based inSolna, SWEDEN
Apealea (paclitaxel micellar) is a patented solvent-free formulation: it applies paclitaxel – a cornerstone within chemotherapy for many different forms of cancer – through Vivesto’s XR-17 technology platform. Apealea is approved by the European regulatory authority EMA for use in combination with carboplatin for the treatment of adult patients with first relapse of ...
Manufactured by:Histocell based inDerio, SPAIN
ASC pre-differentiated to osteoblasts in Histobone scaffold (engineered synthetic bone substitute) for the treatment of bone defects caused by pseudoarthrosis, infections, resections, ...
Manufactured by:3B Pharmaceuticals GmbH based inBerlin, GERMANY
Discovery and preclinical development of peptide drug candidates have been 3BP’s core competencies since the company’s inception. ...
Manufactured by:SHL Medical AG based inZug, SWITZERLAND
The establishment of the Molly platform signifies SHL’s commitment to further addressing the varying needs of our customers and their patients. Introduced in 2010, Molly is SHL’s first preconfigured autoinjector designed to help pharmaceutical companies reduce initial investments and expedite development ...
Manufactured by:Bayer AG based inLeverkusen, GERMANY
Small molecules (SMOLs) have long been in the focus of modern medicine. Over the last century, their advent revolutionized healthcare and improved the lives of patients all over the world. Most of today´s SMOLs exert their function by binding to proteins, thereby modulating their ability to catalyze biochemical reactions. However, a lot of proteins are difficult targets for SMOLs, since ...
Manufactured by:Medicem Group a.s. based inPraha 10, CZECH REPUBLIC
In this area, MEDICEM leverages its patented material platform featuring a unique hydrogel designed to expand under pressure. DILAPAN-S is a synthetic osmotic hygroscopic dilator for the uterine cervix made from proprietary anisotropic xerogel. The embodiment is a sterile synthetic gel rod, which absorbs fluid from the tissue of the cervical canal, resulting in reversible dehydration and ...
Manufactured by:Fresenius SE & Co. KGaA based inBad Homburg v.d.H., GERMANY
FX CorALs Helixone hydro membrane forms a hydrolayer on the inner membrane surface for reduced protein adsorption, resulting in a membrane with low immune response and high selective permeability. 26'27>28-29 Focal points of FX CorAL's development were clinical performance and hemocompatibility, because these two factors belong together in patient centred dialysis. ...
Manufactured by:Biotest AG based inDreieich, GERMANY
Trimodulin (BT588, predecessor BT086) is a human plasma-derived native polyvalent antibody preparation for intravenous administration. Trimodulin contains immunoglobulins IgM (-23%), IgA (~ 21%) and igG (-56%). Trimodulin mediates its mode of action via three potential mechanisms which are; opsonization of causal pathogens, neutralizing of microbial pathogens and their virulence factors (endo- ...
by:Healx Ltd. based inCambridge, UNITED KINGDOM
Our Rare Treatment Accelerator helps academic, patient, and industry groups advance their rare disease drug redevelopment projects. By combining our team, expertise and financial resources with your research and insights, we can get treatments to patients ...
Manufactured by:Medical Wire & Equipment based inCorsham,, UNITED KINGDOM
∑-VCM™ is a preservation and a universal transport media device, used for optimum recovery of viruses, chlamydia, mycoplasma, ureaplasma and Neisseria gonorrhoeae. Antibiotics in the medium inhibit the growth of contaminating bacteria and fungi. It is compatible with culture and molecular diagnostic techniques. ∑-VCM™ can have a positive clinical impact on the development of ...