- Home
- Equipment
- north america
- drug evaluation
Refine by
Drug Evaluation Equipment Supplied In North America
9 equipment items found
by:PSC Biotech® based inNORTH AMERICA
As a leading biotechnology company, MtoZ Biolabs is committed to providing comprehensive, one-stop CRO testing services to scientific research institutions and pharmaceutical companies worldwide. Whether in the early stages of drug discovery, candidate drug evaluation, or preclinical development, MtoZ Biolabs can provide professional technical ...
Manufactured by:ErtelAlsop based inKingston, NEW YORK (USA)
The pharmaceutical and biotech markets require a high level of functional consistency from filtration products. To provide a higher level of confidence for these customers, ErtelAlsop drafted its Drug Master File and submitted it to the FDA Center for Drug Evaluation and Research. ErtelAlsop manufactures all filter pads to very high standards, ...
Manufactured by:BioXcel Therapeutics, Inc. based inNew Haven, CONNECTICUT (USA)
BXCL501 is our most advanced neuroscience clinical program. BXCL501 is a proprietary, sublingual film formulation of dexmedetomidine, a selective alpha-2 receptor agonist. It is being investigated for at-home use for the acute treatment of agitation in bipolar and schizophrenia patients, for agitation in Alzheimer’s disease, and as an adjunctive treatment for major depressive ...
by:Relmada Therapeutics, Inc. based inCoral Gables, FLORIDA (USA)
Novel MOA: REL-1017 works as an NMDAR channel blocker with preferential activity at hyperactive GluN1-GluN2D channels. REL-1017 is a novel N-methyl-D-aspartate receptor (NMDAR) channel blocker in phase 3 clinical trials for the treatment of Major Depressive Disorder (MDD). REL-1017 works with a MOA markedly different from standard serotoninergic ...
Manufactured by:XILIS, Inc. based inDurham, NORTH CAROLINA (USA)
Xilis’ proprietary MicroOrganoSphere (MOS) technology represents a new standard in patient-derived micro-tumor production. From each patient, MOS retains unique patient tissue structure, genetic alterations, gene expression, immune microenvironment and histopathology. Overcoming major limitations of widely used, yet limited, models including xenografts and organoids, MOS offers ...
Manufactured by:Emulate based inBoston, MASSACHUSETTS (USA)
Study lung physiology, disease, and the effect of drug candidates. A more human-relevant model for studying pulmonary physiology, with primary human cells in a lung-specific microenvironment. We are currently developing two distinct models of the human lung—the Alveolus Lung-Chip and the Airway Lung-Chip—to capture the specific tissue architecture and function of ...
Manufactured by:Emulate based inBoston, MASSACHUSETTS (USA)
Evaluate drug candidate toxicity at clinically relevant concentrations in a co-culture human kidney model. Predicting drug-induced kidney toxicity and drug-drug interactions continues to be a challenge due to a reliance on immortalized cell line culture or animal models that do not translate to human response. ...
Manufactured by:BioMark Diagnostics Inc. based inRichmond, BRITISH COLUMBIA (CANADA)
BioMark’s initial liquid biopsy assay detects SSAT1 using an FDA approved drug. SSAT1 is an enzyme with elevated levels in numerous cancers. The assay targets a well researched and understood metabolic pathway. BioMark of a few unique companies using safe therapeutic agents for diagnostic indication. As a non-invasive cost effective red alert tool, it is valuable to physicians around the ...
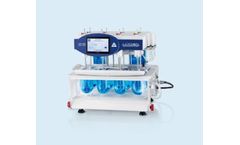
Manufactured by:LOGAN Instruments Corp based inSomerset, NEW JERSEY (USA)
They also feature tight vessel seals to minimize media evaporation and have individual temperature sensors for each vessel, allowing precise monitoring and recording. This design focuses on enhancing the efficiency and accuracy of drug research and the evaluation of generic ...