- Home
- Equipment
- united kingdom
- open surgery
Show results for
Refine by
Open Surgery Equipment Supplied In United Kingdom
7 equipment items found
Manufactured by:Becton Dickinson and Company (BD) based inFranklin Lakes, NEW JERSEY (USA)
TissuePatchSF™ is indicated for use to seal and reinforce against fluid (including cerebrospinal fluid (CSF)) and/or blood leakage where repair of the dura mater is required, air leakage in thoracic surgery (open and minimally invasive) and leakage of low pressure or oozing bleeding or fluid leakage following surgical procedures on soft tissue. ...
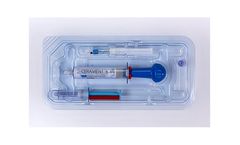
Manufactured by:OrthoPediatrics Corp. based inWarsaw, INDIANA (USA)
Calcium sulfate acts as a resorbable carrier for hydroxyapatite, and hydroxyapatite is highly osteoconductive, promoting bone ingrowth. The characteristics of CERAMENT BONE VOID FILLER make it ideal for minimally invasive surgery and open procedures where bone remodeling is ...
Manufactured by:JUNE Medical USA Inc based inNew Castle, DELAWARE (USA)
JUNE Medical is delighted to announce the launch of the Galaxy II® LUX Connect – the worlds’ first self-retaining ring retractor with light. This unique partnership provides surgeons with a revolutionary solution, giving them better access and a clearer view of the surgical site, and eliminating the disadvantages of using overhead lighting or personal ...
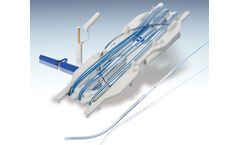
Manufactured by:APC Cardiovascular Ltd. based inHartford, UNITED KINGDOM
Myo-Pace heart wires are a range of myocardial heart wires designed specifically for use as a temporary cardiac pacing wire after open heart surgery. The polyethylene insulation provides the smooth surface necessary to minimise tissue trauma both on placing and withdrawal of the heart wire, whilst the multifilament braided stainless steel wire construction ...
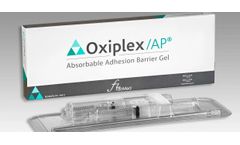
Manufactured by:JUNE Medical USA Inc based inNew Castle, DELAWARE (USA)
An effective adhesion barrier for intrauterine or peritoneal surgery, Oxiplex/AP® Absorbable Adhesion Barrier Gel helps reduce the incidence, extent, and severity of postoperative ...
Manufactured by:Destiny Pharma plc based inBrighton, UNITED KINGDOM
A Phase 2b clinical study, which was a multi-centre, randomized, placebo-controlled study of XF-73 nasal gel as a new product for the prevention of the incidence of post-surgical infections such as methicillin-resistant Staphylococcus aureus (MRSA) completed recruitment at the end of 2020 and top-line results were reported in Q1 2021, as ...
by:SI-BONE, Inc. based inSanta Clara, CALIFORNIA (USA)
Minimally invasive SI joint surgery is the current medical standard of care for SI joint fusion to relieve sacroiliac joint pain. The iFuse Implant System®, available since 2009, is a minimally invasive surgical (MIS) option designed to provide immediate sacroiliac (SI) joint stabilization and allow long-term ...