Refine by
Pcr Sample Equipment & Supplies
19 equipment items found
Manufactured by:Genabio Diagnostics Inc. based inBedford, MASSACHUSETTS (USA)
Intended use: Genabio® Molecular Panel for Vaginitis detects over 30 different pathogens that causes various vaginal symptoms or sexually transmitted ...
Manufactured by:Forefront Medical Technologies based inSingapore, SINGAPORE
Clinically-proven to be effective sample collection; HSA, USFDA & CE registered; Flexible biocompatible nylon for improved comfort; Safe Break Point feature for quick sample ...
Manufactured by:Clinical Diagnostics, a division of R-Biopharm AG based inDarmstadt, GERMANY
RIDA®UNITY combines sample extraction, PCR setup, and real-time PCR into one workflow for maximum walk-away time. ...
Manufactured by:Molbio Diagnostics Pvt. Ltd. based inVerna, INDIA
Intended Use: Quantitative detection and diagnosis of Plasmodium falciparum in human blood specimen and aids in the diagnosis of infection with Malaria caused by Plasmodium ...
Manufactured by:Molbio Diagnostics Pvt. Ltd. based inVerna, INDIA
Intended Use: Quantitative detection Plasmodium vivax and Plasmodium falciparum in human blood specimen and aids in the diagnosis of infection with Malaria caused by Plasmodium vivax and/or Plasmodium ...
Manufactured by:SomaGenics, Inc. based inSanta Cruz, CALIFORNIA (USA)
miR-ID is a novel platform for detecting miRNA using a circularization-based RT-qPCR method. This technology allows SNP discrimination at any position in miRNAs and other small RNAs. miR-ID® is highly sensitive and uses affordable single-dye detection. The technology works well with all sample sources, including total RNA, cell lysates, and tissue ...
by:Diasorin based inSALUGGIA, ITALY
Sample-to-answer, real-time PCR systems designed for improved laboratory efficiency and workflow. ARIES and ARIES M1 Systems are Luminex’s sample-to-answer, real-time PCR systems that are crafted to increase laboratory efficiency, ensure result accuracy, and fit seamlessly into today’s lean ...
Manufactured by:Spark Mobile Diagnostics based inJamnagar, INDIA
Spark Diagnostics is pleased to introduce a Rapid Whole Blood Test for COVID-19IgG / IgM: Cov-CHECK. The test is designed for the rapid qualitative detection of IgG and IgM antibodies to the 2019 novel Coronavirus (2019-nCoV,SARS-CoV-2) in human whole blood, serum or plasma. Cov-CHECK™ – COVID-19 IgG / IgM Test Device is a supplemental rapid screening tool for symptomatic or ...
by:Lytech Co. Ltd based inMoscow, RUSSIA
Reagent Kit POLYVIR SARS-CoV-2 is intended for qualitative detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA by real-time reverse transcription-polymerase chain reaction (real-time RT-PCR) in nucleic acid samples, isolated from clinical samples (nose, nasopharyngeal and oropharyngeal swabs, bronchoalveolar lavage, ...
Manufactured by:BMP Medical based inSterling, MASSACHUSETTS (USA)
We work closely with our customers to provide support on the design and the ability to manufacture their products. IVD parts we produce include lateral flow filtration devices, Polymerase Chain Reaction (PCR) vessels, and sample transport containers. The key to manufacturing an IVD device is color. Many of the IVD tests that these devices are used for require a ...
by:Xiamen Spacegen Co., Ltd. based inxiamen, CHINA
Detection of specific gene sequences in monkeypox virus ...
Manufactured by:OPTI Medical Systems, Inc. based inRoswell, GEORGIA (US) (USA)
OPTI Medical Systems has received US FDA Emergency Use Authorization and CE mark for its OPTI SARS-CoV-2 RT-PCR Test for detection of the virus causing COVID-19. The OPTI SARS-CoV-2 RT-PCR Test is based on real-time reverse transcription polymerase chain reaction (RT-PCR), which provides detection of the viral RNA in the sample. ...
Manufactured by:EntroGen, Inc. based inWoodland Hills, CALIFORNIA (USA)
EntroGen NGS Targeted Hotspot Panel is a comprehensive pan-cancer assay designed to detect clinically relevant hotspot mutations in solid tumors. Utilizing a robust next-generation sequencing (NGS) platform, labs are able to simultaneously analyze several different tumor types by batching up to 12 samples on a single run*. NGS Targeted Hotspot Panel (THSP) is compatible with fresh frozen, and ...
Manufactured by:Lumos Diagnostics based inSarasota, FLORIDA (USA)
CoviDx™ SARS-CoV-2 Rapid Antigen Test is a lateral flow assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasal swabs and nasopharyngeal swabs from patients suspected of a Coronavirus-19 (COVID-19) infection by their healthcare provider within the first 5 days of ...
Manufactured by:3DHISTECH Ltd. based inBudapest, HUNGARY
TMA Master II is the smallest fully automated tissue microarrayer on the market, it easily fits any laboratory ...
Manufactured by:EntroGen, Inc. based inWoodland Hills, CALIFORNIA (USA)
BRCA Complete Expanded Panel is a complete next generation sequencing (NGS) solution for detecting clinically relevant BRCA1 and BRCA2 mutations along with extended coverage of CHEK2, PALB2, RAD51C and TP53. ...
Manufactured by:Co-Diagnostics, Inc. based inSalt Lake City, UTAH (USA)
The Logix Smart™ ZDC (ZIKV-DENV-CHIKV) Test kit uses our patented CoPrimer™ technology to differentiate between the ribonucleic acid (RNA) of Zika, dengue (all 4 serotypes), and chikungunya viruses and to detect and amplify regions of the viruses’ genomes. The test operates using a single step real-time reverse transcriptase polymerase chain reaction (RT-PCR) ...
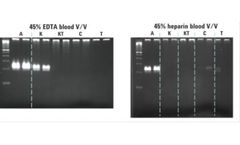
Manufactured by:Agilent Technologies, Inc. based inSanta Clara, CALIFORNIA (USA)
The SureDirect Blood PCR Kit enables direct DNA amplification from many sample types including: liquid blood, dried blood on paper, serum, and plasma. Inhibitor resistance and throughput, common PCR challenges associated with blood samples, are averted through the activity of a newly engineered Taq. Your workflow is streamlined ...
Manufactured by:Nirmidas Biotech, Inc. based inPalo Alto, CALIFORNIA (USA)
The test can be used for a variety of samples including serum, plasma, whole blood, dry blood spot, and saliva in a lab facility. It can be used to assess IgG and IgM levels in a sample with 100% sensitivity and 99.87% specificity without interferences from other diseases. Avidity pGOLD™ assay can tell if a COVID-19 infection is recent or remote greater than 6 months ...