Refine by
Applications
- Artesunate Injection for Malaria
- Artesunate Injection for Malaria Diagnosis of Malaria
- Artesunate Injection for Severe Malaria
- Acute Kidney Injury (AKI) Device for Reduction of Galectin-3 Levels
- Acute Kidney Injury (AKI) Device for Plasmapheresis Therapy
- Acute Kidney Injury (AKI) Device for Patient Selective Apheresis
- Fluid management solutions for COVID-19 challenging cases sector
- Fluid management solutions for surgery sector
- Fluid management solutions for acute kidney injury (AKI) sector
- Fluid management solutions for enhanced recovery sector
- Fluid management solutions for paediatric sector
Renal Injury Equipment & Supplies
97 equipment items found
Manufactured by:CTK Biotech, Inc. based inPoway, CALIFORNIA (USA)
The RaFIA Immunofluorescence Analyzer is a fluorescence immunoassay analyzing instrument intended for use by healthcare professionals to aid in the diagnosis of conditions such as cardiovascular disease, pregnancy, infection, diabetes, renal injury and ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Alpinetin inhibits lipopolysaccharide (LPS)-induced inflammation, activates PPAR-γ, activates Nrf2, and inhibits TLR4 expression to protect LPS-induced renal ...
Manufactured by:Nephria Bio based inPortsmouth, NEW HAMPSHIRE (USA)
Acute Kidney Injury often requires rapid hemodialysis after diagnosis. The NephRedi dialysis cartridge provides toxin removal targeted to address the unique needs of AKI ...
Manufactured by:Alnylam Pharmaceuticals, Inc. based inCambridge, MASSACHUSETTS (USA)
The first and only FDA-approved prescription medication for the treatment of primary hyperoxaluria type 1 (PH1). OXLUMO is a prescription medicine for the treatment of PH1 to lower oxalate in urine in children and ...
Manufactured by:Nephria Bio based inPortsmouth, NEW HAMPSHIRE (USA)
Our NephroMark™ biomarker tool gives patients and providers the ability to track kidney health and recovery after an AKI ...
Manufactured by:Links Medical Products Inc. based inIrvine, CALIFORNIA (USA)
The Silent Knight Hazardous Drug (HD) Safety Seal is a simple yet effective new product that is compliant to the USP 800 Standard of safe medication handling to minimize the risk of exposure to healthcare personnel, patients and the environment. The Silent Knight HD Safety Seal works together with the Silent Knight pouch and Silent Knight pill crushing machine to crush, transport and store ...
Manufactured by:BridgeBio Pharma, Inc. based inPalo Alto, CALIFORNIA (USA)
BBP-711 is an orally-administered small molecule inhibitor of glycolate oxidase (GO) that is being developed to treat conditions of excess oxalate, including primary hyperoxaluria type 1 (PH1) and frequent kidney stone formation. In PH1, loss of function mutation in the AGXT gene results in accumulation of glyoxylate, which is converted into oxalate and leads to kidney stones and organ damage. ...
Manufactured by:bioMérieux S.A. based inMarcy l`Etoile, FRANCE
The NephroCheck Test Kit is a single-use cartridge designed to detect biomarkers of acute kidney injury, TIMP-2 and IGFBP-7. The test provides results in 20 ...
Manufactured by:Eagle Pharmaceuticals, Inc. based inWoodcliff Lake, NEW JERSEY (USA)
Indications: RYANODEX® (dantrolene sodium) for injectable suspension is indicated for the treatment of malignant hyperthermia in conjunction with appropriate supportive measures, and for the prevention of malignant hyperthermia in patients at high ...
by:Homeguard Environmental based inStamford, CONNECTICUT (USA)
High Blood Pressure, Memory and Concentration Problems, Kidney Damage,and Nerve Disorders. It can lead to Abnormal Fetal Development in pregnant women, and can significantly lower a man’s sperm count, leading to a decrease in ...
Manufactured by:InnoSer Belgie NV based inDiepenbeek, BELGIUM
Slow and reproducible cyst formation in the proximal part of the ...
Manufactured by:Eliaz Therapeutics based inSanta Rosa, CALIFORNIA (USA)
Acute Kidney Injury (AKI) and sepsis are major public health concerns, with high incidence, mortality and morbidity. Despite efforts to develop new interventions, current therapeutic options are limited in scope and effectiveness. At Eliaz Therapeutics, we have developed a patented, unique medical device that will remove Galectin-3 (Gal-3) from human blood. Our device removes both bound and free ...
Manufactured by:InnoSer Belgie NV based inDiepenbeek, BELGIUM
Quick progression of cyst formation in the distal segment of the ...
Manufactured by:Nephria Bio based inPortsmouth, NEW HAMPSHIRE (USA)
The kidney healthcare space is multidimensional, ranging from nutrition to hemodialysis. Nephria Bio is first applying our technology to address the needs of individuals with Acute Kidney Injury, or ...
Manufactured by:InnoSer Belgie NV based inDiepenbeek, BELGIUM
Slow progression with cysts in all segments of the ...
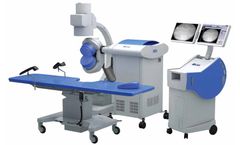
Manufactured by:DirexGroup based inCanton, MASSACHUSETTS (USA)
Duet Magna introduces the combination of electromagnetic technology and the double shockwave concept, allowing for some clear and unique advantages in the field of shockwave ...
Manufactured by:Potrero Medical based inHayward, CALIFORNIA (USA)
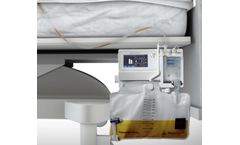
The Accuryn Monitoring System transforms the traditional Foley catheter into a next-generation sensor for accurate, real-time measurement of urine output (UO), intra-abdominal pressure (IAP), and core body temperature (Temp) to help guide ...
Manufactured by:ACIST Medical Systems based inEden Prairie, MINNESOTA (USA)
CVi delivers the power to improve patient safety, minimizing the risk of contrast-induced acute kidney injury (CI-AKI) for coronary catheterizations when compared to manual injection of contrast ...
Manufactured by:Epinex Diagnostics Inc based inTustin, CALIFORNIA (USA)
The Epinex ACR Albumin/Creatinine Ratio Test* is the first quantitative rapid, lateral flow test that measures microalbuminuria, a marker for the early detection of kidney ...
Manufactured by:Cabaletta Bio, Inc. based inPhiladelphia, PENNSYLVANIA (USA)
PLA2R-CAART is being developed to treat patients with PLA2R-associated membranous nephropathy (MN), a B cell-mediated autoimmune disease that affects the kidneys. B cells produce autoantibodies, which are believed to form immune complexes that are deposited at the glomerular basement membrane, causing damage of the filtration barrier, and leading to proteinuria. Over time, ...