Refine by
Surgical Implants Equipment & Supplies
120 equipment items found
Manufactured by:LZQ Tool Co., Ltd. based inPudong New District, CHINA
Temporary abutments are designed to form a gingival sulcus and to preserve papillae during the one stage surgical procedure or after a two stage uncovering procedure. Choose an abutment height that is sufficient to laterally support the interproximal papillae without its being too tall such that it would receive undesirable forces from the tongue. Corresponding and matching ...
Manufactured by:Voyage Medical Solutions based inLongview, TEXAS (USA)
Our VMS team has created partnerships with multiple surgical device companies for our surgeons’ needs. Contact us today for a complete portfolio. If you don’t see a specific implant product you’re looking for, feel free to contact us to see if we can get you the durable medical equipment you ...
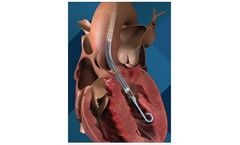
Manufactured by:enVVeno Medical based inIrvine, CALIFORNIA (USA)
The VenoValve is a first-in-class surgically implanted solution being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). Implanted into the femoral vein, the VenoValve is designed to act as a one-way valve to help restore proper blood flow up the leg, to return sufficient blood back to the heart. ...
Manufactured by:SAEYANG CO.,LTD. based inDaegu, SOUTH KOREA
Features: Optic LED Control, Compact Micro motor 40.000 rpm (1:1), 9 memory programs can be stored on single motor, Light & compact optic motor for speedy and accurate ...
Manufactured by:Abiomed based inDanvers, MASSACHUSETTS (USA)
Impella 5.0 is a temporary, minimally invasive heart pump that provides circulatory support, enabling the heart to rest and recover. Impella LD is a surgically implanted heart pump that provides temporary support to the heart during and after a procedure. Impella 5.0 and Impella LD heart pumps allow patients to walk around while on support to improve recovery, if ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Non-bone tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. Non-bone tendons used in ACL reconstruction procedures allow for multiple fixation ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's bone-tendon-bone (BTB) grafts are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These grafts allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Matrix HD Allograft Dermis is an acellular human dermis allograft sterilized to a Sterility Assurance Level (SAL) of 10-6 using the Tutoplast Tissue Sterilization Process. This proprietary process retains the three-dimensional intertwined multidirectional fibers and mechanical properties of the native dermis tissue. The Matrix HD graft provides a natural scaffold to support the body's ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI's Achilles tendons are sterilized through the BioCleanse Tissue Sterilization Process without the use of irradiation. These tendons allow for multiple techniques and fixation options in ligament reconstruction ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Conventional allografts are used for various procedures to fill bony voids or gaps in a patient's skeletal system, restore segmental bone loss due to trauma, osteoporosis, removal of tumors or to aid in the surgical correction for a deformity. These grafts provide a scaffold for bone ingrowth to allow for remodeling with the patient’s own bone. These grafts are pre-cut to select sizes to ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical offers the only meniscal allograft with both bone and meniscus sterilized through the BioCleanse® Tissue Sterilization Process. The BioCleanse processed meniscus graft provides a sterilized alternative to traditional aseptically processed meniscus allografts and sets the standard for safety and ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
RTI Surgical’s fresh-stored osteochondral (OC) allografts enable surgeons to resurface osteochondral defects with mature hyaline cartilage and repair subchondral bone in a single procedure. Fresh grafts are cleansed, processed and preserved to maintain chondrocyte ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Fortiva Porcine Dermis is a non-crosslinked acellular porcine dermal matrix offering a safe and natural biologic option. Fortiva implants are processed through the Tutoplast® Tissue Sterilization Process and terminally sterilized via validated low-dose gamma irradiation to achieve a sterility assurance level (SAL) of 10-6. Fortiva Porcine Dermis is a ready-to-use biologic implant that acts as ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Cortiva Allograft Dermis is a non-crosslinked acellular dermal matrix offering a safe and natural biologic option and sterilized to a sterility assurance level (SAL) of 10-6 through the Tutoplast® Tissue Sterilization ...
Manufactured by:RTI Surgical based inAlachua, FLORIDA (USA)
Whole bones are used as a sterile source of cortical and cancellous bone. These implants may also be used in procedures that restore segmental and critical bone loss in the extremities due to trauma, bone disease, or from the removal of tumors and/or hardware. After being processed through RTI's BioCleanse® Tissue Sterilization Process, they are terminally sterilized in their final packaging ...
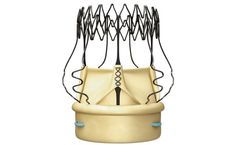
Manufactured by:Corcym UK Limited based inLondon, UNITED KINGDOM
The Perceval Platform is based on a sutureless and collapsible design that simplifies the surgical implantation, reducing the impact of surgery and facilitating faster ...
Manufactured by:KOS LtD based inJUNG-GU, SOUTH KOREA
SPECIFICATIONS: Chemical composition (wt%) : ASTM F2063 - "Standard Specification for Wrought Nickel-Titanium Shape Memory Alloys for Medical Devices And Surgical Implants" ...
Manufactured by:Corcym UK Limited based inLondon, UNITED KINGDOM
Perceval is a pericardial surgical heart valve with a sutureless and collapsible design that simplifies the surgical implantation. Reducing operative trauma and post-operative complications. Enabling faster Patient ...
Manufactured by:R & L Medical based inHangzhou City, CHINA
R&L Medical Calcium Sulphate has obtained NMPA Class III and meets the purity requirements for high-puritycalcium sulfate in surgical implants as specified in ASTM F2224-09, the Standard Specification for High Purity Calcium Sulfate Hemihydrate or Dihydrate for Surgical ...
Manufactured by:TissX based inMinneapolis, MINNESOTA (USA)
The development of percutaneous cardiac valves (aortic, mitral, tricuspid and pulmonary). The development of surgically implanted cardiac valves. Pericardial Patches and other tissue for surgical, vascular and other applications. Functional components for structural heart disease treatment and other vascular uses. ...