Show results for
Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Thrombosis Equipment Supplied In Europe
14 equipment items found
Manufactured by:Mavera Medical Devices Inc. based inAnkara, TURKEY
The Mythica Ultrasonic Thrombosis Cavitation System is designed for the percutaneous treatment of blood clots within the peripheral vasculature, utilizing ultrasound technology for minimal invasive surgery. The system features an external power generator, a piezoelectric handle, and an ultrasonically driven catheter. ...
Manufactured by:TUGI - Promoton s.ro. based inPlzen, CZECH REPUBLIC
TUGIs are approved for adult users only. TUGIs are not intended for unlisted medications (e.g., diabetes medications). TUGIs’ innovation is protected by a number of registered industrial designs, patents, and trademarks in the EU, USA, Canada, Switzerland, Japan, Republic of Korea, India, Norway, Russia, and other countries. The principle, design, and names of these products are protected. ...
Manufactured by:ORGENTEC Diagnostika GmbH is part of the Sebia Group based inMainz, GERMANY
Detection of these autoantibodies is an integral part of the classification criteria issued by the International Society on Thrombosis and Hemostasis. Beta-2-GPI antibodies are associated with the occurrence of arterial or venous thrombosis, as well as with recurring miscarriage. Beta-2-GPI antibodies are considered to have a higher specificity than cardiolipin ...
Manufactured by:ORGENTEC Diagnostika GmbH is part of the Sebia Group based inMainz, GERMANY
This assay contributes to the diagnosis of antiphospholipid syndrome (APS). Regardless of their immunoglobulin class, cardiolipin autoantibodies are an important criterion for the diagnosis of APS. Autoantibodies against cardiolipin are also an immunological classification criterion for systemic lupus erythematosus (SLE). In addition, the quantitative determination of anti-cardiolipin is very ...
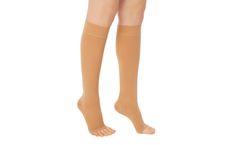
Manufactured by:Variteks Ortopedi Sanayi A.S. based inÇatalca, TURKEY
Indications: Symptoms of spider veins and minor swelling. Heaviness and fatigue of the leg. Mild varicose veins during pregnancy. Prophylaxis of thrombosis and embolism for immobile patients. Prophylaxis during travel. ...
Manufactured by:Variteks Ortopedi Sanayi A.S. based inÇatalca, TURKEY
Indications: Varicose veins with mild oedema. Severe varicose veins during pregnancy. Post superficial and deep venous thrombosis. Post sclerotherapy and venous surgery. Chronic venous insufficiency (CVI). After treatment of light ulcers. Post-thrombotic syndrome (PTS). Post-traumatic oedema. ...
Manufactured by:ELCAT GmbH based inWolfratshausen, GERMANY
Very good sensitivity, Ultrasound stylus probes, Replaceable ultrasound modules, Battery operated with energy management for long operating times and low operating costs. Ultrasound probes (pen probe), 2 MHz CW (fetal), 4 MHz CW, 8 MHz CW (option: intraoperative), Headphone connection, Battery operation with energy management, Long operating times, Low operating costs, The handydop works with ...
Manufactured by:Tcoag Ireland Ltd based inBray, IRELAND
TriniLIA™ D-Dimer II is intended for the quantitative determination of D-Dimer in plasma by the immunoturbidimetric method. It can be used to aid in the diagnosis of exclusion of deep vein thrombosis (DVT) and pulmonary embolism (PE) ...
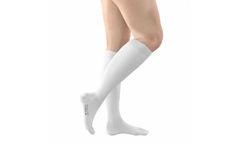
Manufactured by:G&N Medical based inHorsham, UNITED KINGDOM
FITLEGS™ AES (anti-embolism stockings) help to prevent the formation of Deep Vein Thrombosis (DVT) in the legs by applying clinically proven graduated compression.* FITLEGS™ AES are designed to be simple to fit and apply, which reduces sizing errors and nursing time. G&N has developed an effective grip sole and applied it to our leading brand FITLEGS™ to ...
Manufactured by:g.tec medical engineering GmbH based inSchiedlberg, AUSTRIA
The g.Estim FES is a programmable functional electrical stimulator (FES) and is intended for neurological therapeutic applications such as temporary reduction of muscle spasms, prevention or retardation of disuse atrophy, increasing local blood flow in the treatment area, muscle re-education, prevention of post-surgical phlebo-thrombosis through immediate stimulation of calf ...
Manufactured by:I-Tech Medical Division based inMartellago (VE), ITALY
Power Q-1000 Premium is a compressive therapy device with 6 inflating modes, ideal for treating lymphedema, early-stage cellulite, post-mastectomy arm drainage, rheumatoid arthritis, hematomas following surgery and post-operative prophylaxis in venous thrombosis ...
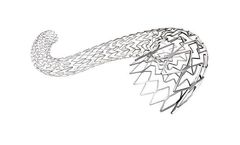
Manufactured by:Bentley InnoMed GmbH based inHechingen, GERMANY
Thrombosis is known to be a wide spread disease. Venous stenting is proven to have a positive impact on the quality of life, especially of young and active people. The BeYond venous stent is designed to respect the natural venous anatomy. It offers the optimal balance between radial force, flexibility and deployment ...
Manufactured by:Becton Dickinson and Company (BD) based inFranklin Lakes, NEW JERSEY (USA)
This could allow for local drug elution while leaving no permanent residual polymer, which might address the issue of late stent thrombosis with current DESs. In addition, Tepha is developing fully absorbable polymer stents containing TephaFLEX® material, which would provide the necessary support during the vessel remodeling period and then completely ...
Manufactured by:Arterius Limited based inLeeds, UNITED KINGDOM
ArterioSorb is produced from the FDA approved polymer, PLLA, which is already universally approved for use in cardiovascular stents, sutures and orthopaedic applications. ArterioSorb is a tubular stent with closed-cell (with connectors for strength) and open-cell (with fewer connectors for flexibility) modules and increasing cell size as one moves away from the centre: The smaller cells at the ...