Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Tumor Response Equipment Supplied In Usa
42 equipment items found
Manufactured by:IN8Bio Inc. based inNew York, NEW YORK (USA)
INB-200, our DeltEx DRI, is a genetically modified autologous gamma-delta T cell product candidate for the treatment of solid tumors. This is the first genetically engineered gamma-delta T cell therapy to be administered to patients and our initial indication is newly diagnosed Glioblastoma (GBM). Since 2005 the standard of care treatment for GBM has been surgical resection ...
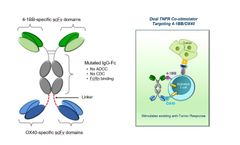
Manufactured by:Aptevo Therapeutics based inSeattle, WASHINGTON (USA)
Dual targeting of 4-1BB and OX40 provides synergistic co-stimulation of T cells with the potential to amplify the cytotoxic function of activated T cells and NK cells, potentially leading to more robust anti-tumor ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
BMS-599626 Hydrochloride displays ~8-fold less potent to HER4 (IC50=190 nM), >100-fold to VEGFR2, c-Kit, Lck, MEK. BMS-599626 Hydrochloride inhibits tumor cell proliferation, and has potential to increase tumor response to ...
Manufactured by:Tonix Pharmaceuticals Holding Corp. based inChatham, NEW JERSEY (USA)
TNX-1700 is a stabilized recombinant version of Trefoil Factor 2 (TFF2) and is being developed by Tonix as a biologic to treat gastric and colorectal cancers. TFF2 is a small, secreted protein, encoded by the TFF2 gene in humans that is expressed in gastrointestinal mucosa where it functions to protect and repair mucosa. TFF2 is also expressed at low levels in splenic immune cells and is now ...
by:Myeloid Therapeutics based inCambridge, MASSACHUSETTS (USA)
Myeloid is focused on advancing its ATAK CAR monocytes, which are myeloid cells with innate immune receptor-inspired CARs to recognize and kill cancer. Additionally, Myeloid has streamlined manufacturing for its ATAK cell therapy candidates through a rapid, single-day cell process. This method provides significant advantages to the patient, contract development, and manufacturing organizations ...
Manufactured by:MedChemExpress LLC (MCE) based inMonmouth Junction, NEW JERSEY (USA)
Autophagy is an intracellular degradation system that delivers cytoplasmic constituents to the lysosome. Autophagy plays a wide variety of physiological and pathophysiological roles. Different selective forms of autophagy have been identified and characterized, leading to the specific degradation of organelles or pathogens. These selective pathways include the autophagic degradation of ...
Manufactured by:Alphageneron based inCambridge, MASSACHUSETTS (USA)
Our lead clinical stage natural killer (NK) cell therapy, is an autologous platform technology that activates NK cells to kill cancers expressing a unique membrane form of Heat Shock Protein 70 ...
Manufactured by:Celularity Inc. based inFlorham Park, NEW JERSEY (USA)
CYNK-CAR candidates are being developed as allogeneic, off-the-shelf approaches through gene modification of human placental hematopoietic stem cell-derived natural killer (NK) cells. Various CAR constructs against hematological and solid tumor targets are under pre-clinical ...
Manufactured by:Theriva Biologics, Inc., formely known as ynthetic Biologics, Inc. based inRockville, MARYLAND (USA)
Building on the clinical advancement of VCN-01, Theriva developed the Albumin Shield™ technology to protect oncolytic viruses from circulating anti-oncolytic virus antibodies after systemic administration. We anticipate that this technology will enable multiple oncolytic virus doses to be administered in therapeutic cycles to treat particularly refractory ...
Manufactured by:MedGenome Inc. based inCounty of New Castle, DELAWARE (USA)
OncoPeptTUME — A novel in-silico approach to model the tumor microenvironment and predict treatment efficacy and long-term survival benefits for immunotherapy applications. Cancer immunotherapy is now established as a major therapeutic modality. Cancer immunotherapy drugs elicit their anti-tumor immune response in a subset of the treated ...
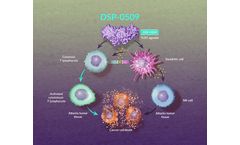
by:Sumitomo Pharma Oncology, Inc. based inCambridge, MASSACHUSETTS (USA)
DSP-0509 is an investigational Toll-like receptor (TLR) 7 agonist, which is hypothesized to induce cytokine production, activate cytotoxic T lymphocytes, and sustain immune-mediated antitumor ...
Manufactured by:RedHill Biopharma Ltd. based inTel-Aviv, ISRAEL
RHB-107 (INN: upamostat) (formerly MESUPRON) is a first-in-class, once-daily, orally-administered, potent inhibitor of serine proteases targeting multiple indications, including COVID-19, cancer, inflammatory lung diseases and gastrointestinal diseases. ...
Manufactured by:Senti Biosciences based inSouth San Francisco, CALIFORNIA (USA)
Natural Killer (NK) cells are essential components of the immune system, playing an important role in the body’s defensive response against tumors and infected cells. NK cells constantly patrol the body to detect and neutralize foreign cells. Senti Bio believes there are several advantages that NK cells confer in relation to other potential immune cell ...
Manufactured by:Kaio Therapy, LLC based inRaleigh, NORTH CAROLINA (USA)
Kaio Therapy's Immune Activation Heat Therapy is a novel hyperthermia system that delivers precisely controlled heat at the cellular level within a tumor. Because Kaio’s IAHT elegantly induces necrosis while activating a systemic, tumor specific response, it can also serve as a platform for other immunotherapies seeking higher efficacies, ...
Manufactured by:Actym Therapeutics based inBerkeley, CALIFORNIA (USA)
Actym Therapeutics, based in Berkeley, CA, is a privately held biotechnology company focused on the discovery and development of novel immunotherapies to treat cancer. The company has developed an attenuated, microbial-based, technology platform called STACT (S. Typhimurium-Attenuated Cancer Therapy). In preclinical studies, STACT specifically enriches in many types of solid tumors and not in ...
Manufactured by:INOVIQ Ltd based inNotting Hill, AUSTRALIA
INOVIQ has three autoantibody tests in development for early detection of ovarian, breast and lung cancers. BARD1 autoantibody tests measure autoantibodies to variant BARD1 proteins in the blood and use a proprietary cancer-specific algorithm to combine these levels into a cancer score that identifies the presence or absence of a specific cancer. BARD1 autoantibodies reflect the body’s ...
Manufactured by:Veracyte based inMarseille Cedex 9, FRANCE
Designed to deliver critical information to guide chemotherapy decision-making, Immunoscore® can be used in Stage II localized colon cancer to help physicians identify patients who may be spared adjuvant chemotherapy, and in Stage III localized colon cancer to inform them about the optimal treatment duration for their patients. ...
Manufactured by:Brooklyn ImmunoTherapeutics (BTX) based inSan Diego, CALIFORNIA (USA)
Cytokines are powerful immune factors that play an essential role in T cell signaling, activation, proliferation, and tumor-fighting responses. We know cytokines may induce beneficial reactions in cancer patients. To date, available cytokine therapies have been recombinant and have historically employed high doses. These therapies are often associated with ...
Manufactured by:Fate Therapeutics, Inc. based inSan Diego, CALIFORNIA (USA)
The oncology therapeutic landscape has been transformed with the use of checkpoint inhibitor therapy. Despite the clinical benefit conferred by approved checkpoint inhibitor therapy against a variety of tumor types, these therapies are not curative and, in most cases, patients either fail to respond or their disease progresses on these agents. Resistance to checkpoint inhibitor ...
by:Starton Therapeutics based inParamus, NEW JERSEY (USA)
STAR-LLD is in development in two continuous delivery systems: subcutaneous and transdermal. STAR-LLD is in development for new multiple myeloma indications for lenalidomide and achievement of superiority versus oral lenalidomide in maintenance treatment of multiple myeloma. The STAR-LLD delivery system is expected to provide significant reductions in discontinuations and treatment abandonment by ...