- Home
- Equipment
- asia middle east
- aortic stented tissue valve
Show results for
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Aortic Stented Tissue Valve Equipment Supplied In Asia Middle East
9 equipment items found
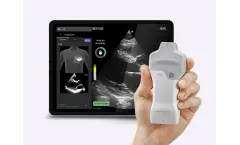
Manufactured by:Vscan Rocks, by GE HealthCare based inSuzhou, CHINA
Providing rapid echocardiographic assessments at the point of care can be difficult with time and resource constraints. Caption AI helps you overcome those challenges. With this powerful and easy-to-use tool, you can bring focused echo to any patient anytime – and get them on the right care path ...
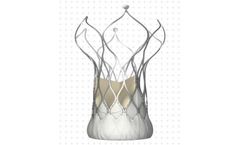
Manufactured by:MicroPort Scientific Corporation based inShanghai, CHINA
VitaFlow Aortic Valve, uses bovine pericardial leaflets to provide enhanced valve durability, its innovative frame and skirt designs deliver reliable hemodynamic performance, and dependable clinical outcomes for ...
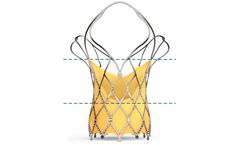
Manufactured by:Biosensors International Group, Ltd. based inSingapore, SINGAPORE
The ALLEGRA Transcatheter Heart Valve integrated with the IMPERIA Delivery System offers a state-of-the-art solution for transcatheter aortic valve interventions. Designed to maintain hemodynamic stability throughout deployment, the Permaflow Delivery System allows for continuous blood flow, providing medical professionals with the time needed to accurately position the device without stress. The ...
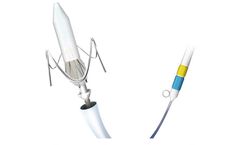
Manufactured by:Sahajanand Medical Technologies Ltd. based inSurat, INDIA
Self-expanding TAVI device to have 2 rows of marker; First Markers are located at Node 1. Second Markers are located at Node 3. First marker helps; In precise implantation of the valve at the targeted implantation zone. To ascertain the depth of implant. Second marker indicates; When the THV leaflets are going to get ...
Manufactured by:Genesis MedTech based inSingapore City, SINGAPORE
J-Valve system: a new generation TAVI Device with 3 claspers. 3 Claspers deployed at the bottom level of Valsalva sinus. CValve migrating to the propriate position with guidance of the claspers. DJ-Valve® self-expanded and system completely deployed. EJ-Valve® fixed in annulus level and delivery device ...
Manufactured by:Relisys Medical Devices Limited based inRanga Reddy, INDIA
The VIENNA AORTIC VALVE SE self-expandable transcatheter valve is designed to treat severe Aortic stenosis in patients at medium to high risk for open heart surgery. The minimal invasive heart valve replaces the native valve without the need for concomitant surgical removal and is placed via the femoral access. The new generation device comes fully pre-mounted with specially prepared bovine ...
Manufactured by:Biosensors International Group, Ltd. based inSingapore, SINGAPORE
The ALLEGRA Transcatheter Heart Valve (THV) is engineered for treating severe calcified aortic valve stenosis, focusing on maintaining optimal hemodynamic performance with single-digit mean pressure gradients and expansive effective orifice areas. Designed for high-risk patients, the ALLEGRA leverages a unique self-expanding ...
Manufactured by:Best Hope Precision Device Co., Ltd based inJiaxing City, CHINA
Aortic stenosis is a common disease where one of the heart valves narrows, restricting the flow of blood into the body. When it gets bad enough, the diseased valve often needs to be ...
Manufactured by:Magenta Medical Ltd. based inKadima, ISRAEL
The Magenta percutaneous Left Ventricular Assist Device (pLVAD) is a self-expanding percutaneous catheter-mounted arterial pump that is intended to provide temporary mechanical circulatory support for the left ventricle (LV) during high-risk percutaneous coronary interventions (HR-PCI) and in patients hospitalized with cardiogenic shock (CS). In the course of HR-PCI and CS, the LV may require ...