Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Hospitalization Rate Equipment Supplied In Europe
5 equipment items found
Manufactured by:Pergo Medikal ve Ilac Sanayi AS based inBuca, TURKEY
Reduces hospital infection rates. Offers additional sterile protection during procedures. Available in different lengths for complete coverage from transducer array to connector. Easy opening with special design for correct probe inserting. Guarding expensive imaging equipment during operation. ...
Manufactured by:Artivion, Inc based inKennesaw, GEORGIA (US) (USA)
Type A Aortic Dissection (TAAD) presents itself emergently. Left untreated, mortality of type A dissection is reported to be approximately 1% to 2% per hour after onset of symptoms1 and can lead to 50% mortality in the first 48 ...
Manufactured by:Natus Medical Incorporated (Natus) based inPleasanton, CALIFORNIA (USA)
With its minimal size and medical grade all-in-one PC, the BRAIN QUICK ICU Line is ideal for EEG monitoring in the ICU and NICU. The system features an intuitive Hospital Grade, IP54 rated touch screen and the ability to set up EEG display and trend analysis in addition to event management over a network. Continuously monitor 29, 38 or 48 channels of EEG ...
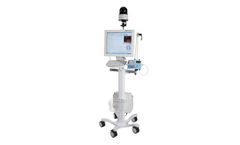
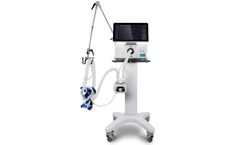
Manufactured by:heyer medical AG based inBad Ems, GERMANY
VG55 is specially designed for Non-Invasive Ventilation (NIV). Research has shown that early application of NIV can effectively decrease intubation rates and hospitalization length. The application of NIV can also prevent ventilator-associated pneumonia (VAP), as well as promote respiratory care. VG55 adopts advanced turbine technology, provides NIV with ...
Manufactured by:Destiny Pharma plc based inBrighton, UNITED KINGDOM
A Phase 2b clinical study, which was a multi-centre, randomized, placebo-controlled study of XF-73 nasal gel as a new product for the prevention of the incidence of post-surgical infections such as methicillin-resistant Staphylococcus aureus (MRSA) completed recruitment at the end of 2020 and top-line results were reported in Q1 2021, as ...