Refine by
Locations
- Europe
- Albania
- Andorra
- Austria
- Belarus
- Belgium
- Bosnia & Herzegovina
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Faroe Islands
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Kosovo
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Macedonia
- Malta
- Moldova
- Monaco
- Montenegro
- Netherlands
- Northern Ireland
- Norway
- Poland
- Portugal
- Romania
- San Marino
- Serbia
- Slovakia
- Slovenia
- Spain
- Sweden
- Switzerland
- Turkey
- Ukraine
- United Kingdom
- Vatican City
Replacement Surgery Equipment Supplied In Europe
16 equipment items found
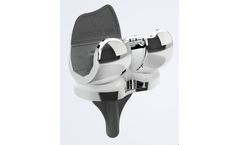
Manufactured by:Medacta International based inCastel San Pietro, SWITZERLAND
AMIS (Anterior Minimally Invasive Surgery) is the only hip replacement technique that follows both an inter muscular and inter nervous path to greatly decrease damage to periarticular structures while preserving tissue. AMIS is a true muscle-sparing procedure supported by the industry’s most detailed surgeon training protocol. ...
Manufactured by:Smith & Nephew plc based in, UNITED KINGDOM
The ANTHOLOGY system is a hip stem (femoral implant with a neck and ball) used for primary hip replacement surgery. The hip joint is a ball and socket joint, connecting the femur with the pelvis. The head of the femur, shaped like a ball, fits tightly into the acetabulum, forming the ball and socket joint of the hip, allowing the leg to move forward and ...
Manufactured by:Surgival based inPaterna, SPAIN
Designed to address specific anatomical needs, these stems provide robust performance without compromising bone integrity, critical for successful hip replacement ...
Manufactured by:Microport Orthopedics Inc. based inArlington, TENNESSEE (USA)
The Profemur® line of primary hip stems has an established clinical history. Each Profemur® hip stem offers its own philosophy to provide fixation and replace bone anatomy. Triple taper stem philosophy" Designed for primary hip replacement surgery, the Profemur® Gladiator® hip stem is a triple tapered wedge stem with a distinct ...
Manufactured by:Heraeus Medical GmbH based inWehrheim, GERMANY
Its distinctive green color is due to the inclusion of colorant E141. This product offers a broad spectrum of activity against pathogens that cause infections post-hip and knee replacement surgeries. The simultaneous release of gentamicin and clindamycin addresses up to 90% of pathogens found in prosthetic joint infections (PJI). It provides an initial high local ...
Manufactured by:Sysmex Partec GmbH based inGoerlitz, GERMANY
Axillary management and the relevance of sentinel lymph node biopsy (SLNB) have undergone major changes in the last years. Clinical trials, which have investigated less invasive treatment approaches as a replacement of axillary surgery, indicate that axillary dissection can be omitted in patients with limited metastatic involvement. Consequently, treatment ...
Manufactured by:Baymed Inovative Health Products Inc. based inMerkez/Kilis, TURKEY
Baymed® disposable cardiovascular drapes provide the strength, versatility and efficiency needed for the critical demands of cardiovascular surgery. Baymed® disposable cardiovascular drapes, made with heavy-duty fabric and Advanced Bayteks® Reinforcement fabric for fluid management in fenestrations, may eliminate the need for costly drape layering. Baymed® ...
Manufactured by:Active Implants LLC based inMemphis, TENNESSEE (USA)
The NUsurface® Meniscus Implant is a meniscus replacement in patients with persistent knee pain following medial meniscus surgery. Meniscus replacement has the potential to fill the gap between minimally invasive meniscus repair and total knee replacement. The implant is made from polycarbonate-urethane (PCU) – a ...
Manufactured by:Conformis based inBillerica, MASSACHUSETTS (USA)
iTotal PS is the only truly custom-made posterior stabilized total knee replacement, designed ...
Manufactured by:LINXobere Medizintechnik GmbH based inBremen , GERMANY
Our product portfolio includes more than 300 different needle types and more than 100,000 different thread-needle combinations to meet the requirements of the surgical procedure and the surgeon’s personal ...
Manufactured by:Biovision GmbH based inIlmenau, GERMANY
BetaBASE is a bioresorbable bone replacement from microporous and macroporous β-tricalcium phosphate. BetaBASE consists of pure-phase β-TCP. Potential risks of infection involved in the use or processing of biological material (HIV, BSE, inter alia) are non-existent with BetaBASE. BetaBASE has an interconnected pore system with micropores and macropores that reproduce the well-known ...
Manufactured by:TUR GmbH based inRostock, GERMANY
kryotur is the traditional conducting cryotheray integrated in the physiotur Symphony therapy tower. It can be combined with kimatur, the radial shockwave therapy, as well as with TUR electrotherapy and ultrasound therapy models. The cold energy is achieved by meand of thermo-electrical modules (Peltier elements) that cool a fluid down to a preset temperature. The fluid then circulates and ...
Manufactured by:Biovision GmbH based inIlmenau, GERMANY
BioBASE is a bioresorbable bone replacement from microporous and macroporous α-tricalcium phosphate. Biobase is an inorganic, bioresorbable bone replacement from pure-phase α-tricalcium phosphate. BioBASE is a bone replacement for temporarily filling pathological, traumatic and postoperative bone defects. Biobase has a system of micropores (< 5 μm) and macropores (max. 1 mm) ...
Manufactured by:MicroPort Scientific Corporation based inShanghai, CHINA
Skywalker™ is a robotics system independently developed by MicroPort to carry out total knee arthroplasty (TKA), completed First In Man Clinical Trial (FIM) at Ninth People’s Hospital Shanghai on June 30th 2020. Director Li Huiwu and his team successfully completed the operation on a female patient to restore her lower limb ...
Manufactured by:SYMATESE based inCHAPONOST, FRANCE
COLLAPAT®II is a collagen medical device containing hydroxyapatite used as bone substitute in orthopaedics, spine surgery and in maxillo facial surgery and odonto-stomatology. COLLAPAT® II is a bone void filler presented in a sponge form. It is composed of a collagen structure in which ceramised hydroxyapatite granules are dispersed. The granules of hydroxyapatite give the material its ...
Manufactured by:emka Technologies S.A.S based inParis, FRANCE
easyTEL+ is a fully implantable digital system, that transmits physiological data from conscious freely moving laboratory animals. It can be remotely managed and configured. ...