- Home
- Equipment
- north america
- catheter device
Show results for
Refine by
Catheter Device Equipment Supplied In North America
36 equipment items found
Manufactured by:Resolution Medical based inFridley, MINNESOTA (USA)
PTFE, Urethane, Tungsten, Gold). The manual coil winder is the perfect machine for transferring reinforcing wire on to catheter ...
Manufactured by:SRS Medical based inN. Billerica, MASSACHUSETTS (USA)
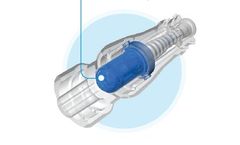
Knowing when to remove a Foley has always been a challenge. Doing it without disturbing the catheter was impossible… until now. Attaching directly to an existing Foley catheter, the device works by using the catheter balloon to measure changes in vesical pressure during both the filling and emptying ...
Manufactured by:Medcomp based inHarleysville, PENNSYLVANIA (USA)
Silicone Hemo-Cath devices provide a biocompatible and flexible design, which may reduce kinking and vessel damage. Hollow Fast Track™ Stylet enhances OTW insertion for less traumatic and fast insertion. MRI SAFE. ...
Manufactured by:Duke Extrusion based inMorgan Hill, CALIFORNIA (USA)
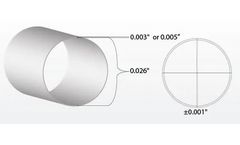
The 35D Pebax™ Stock Single Lumen Tubing offers specific dimensions with an inner diameter of 0.016 inches and an outer diameter of 0.026 inches, catering to precise medical applications. This tubing is widely utilized in medical devices due to its superior flexibility and durability. Known for its ability to withstand various environmental factors, the Pebax™ polymer ...
Manufactured by:Prytime Medical Devices, Inc. based inBoerne, TEXAS (USA)
2 Introducer Sheaths, 5 Needles, 6 Syringes, 2 Catheter Securing Devices, 1 Set Average Zone 1 & Zone 3 Tubing ...
Manufactured by:Pepex Biomedical, Inc. based inNorcross, GEORGIA (US) (USA)
According to the Centers for Disease Control and Prevention (CDC), Sepsis is the tenth most common cause of death overall in the U.S. Sadly, there is no commercially available test that actually detects the sepsis bacteria and current clinical protocols are simply a process of ...
Manufactured by:ClariVein Powered by Merit Medical based inSouth Jordan, UTAH (USA)
The catheter is easily identified under vascular imaging and incorporates a cartridge for secure fastening to the Motor Drive ...
Manufactured by:Nextern Inc based inWhite Bear Lake, MINNESOTA (USA)
We specialize in the design, development, and manufacturing of complex, highly specialized catheters, steerable devices, and delivery systems for interventional markets. We’ve worked with partners on steerable balloon catheters, steerable neurovascular devices, and delivery devices for structural heart ...
Manufactured by:Sanrai International based inEndicott, NEW YORK (USA)
The Bedal DrainPatch is a new drainage catheter fixation device that provides significantly more adhesive strength while still being easy to remove. The Bedal Drain Patches are a range of catheter stabilization devices designed to accommodate a wide range of diameters with only one product. The unique system provides a watertight ...
Manufactured by:Practivet, Inc. based inTempe, ARIZONA (USA)
The first and only FDA-cleared device shown to significantly reduce all types of reflux into a catheter and feature ICU Medical's proven infection control ...
Manufactured by:Attwill Medical Solutions based inLodi, WISCONSIN (USA)
Anti-thrombin Heparin (ATH) technology is a breakthrough heparin formulation that reduces the risk of blood clots and unwanted thrombosis in catheters and other medical devices, but is safer and more active than other Heparin on the market. ATH is a connector device that delivers novel antithrombin and heparin (anticoagulant) into the inner ...
Manufactured by:Dynarex Corporation based inOrangeburg, NEW YORK (USA)
Dynarex Resp-O2 Closed Suction Trach Catheters are disposable respiratory devices designed to safely remove secretions from the respiratory tracts of ventilator-dependent patients. The device allows ventilation to continue during suctioning, helps prevent cross-contamination, and protects caregivers from airborne ...
Manufactured by:Omega Medical Imaging, LLC. based inSanford, FLORIDA (USA)
Elevate your program to the next level of dose reduction and ...
Manufactured by:Cirtec Medical based inBrooklyn Park, MINNESOTA (USA)
Long known for providing complete outsourcing solutions for Class II and III medical devices, Cirtec is pleased to announce we are now producing continuous draw Nitinol tubing. We understand that designing devices in a highly regulated industry can be challenging and our experienced and knowledgeable Nitinol team is here to help. We provide more than just Nitinol tubing, we provide vertically ...
Manufactured by:Thrombolex, Inc. based inNew Britain, PENNSYLVANIA (USA)
The BASHIR S-B Endovascular Catheter is a device intended for the localized infusion of physician-specified fluids, including thrombolytics, into the peripheral ...
Manufactured by:Biomerics based inSalt Lake City, UTAH (USA)
We specialize in the design, development, and manufacturing of complex, highly specialized catheters, steerable devices, and delivery systems for interventional markets. We’ve worked with customers on steerable balloon catheters, steerable neurovascular devices, and delivery devices for structural heart ...
Manufactured by:Viant Medical based inFoxborough, MASSACHUSETTS (USA)
Complex interventional devices. Steerable, balloon, and RF ablation catheters. Closure devices (femoral arterial and venous access). Introducers for transradial artery access. ...
Manufactured by:Dynarex Corporation based inOrangeburg, NEW YORK (USA)
Dynarex Resp-O2 Closed Suction Endotracheal Catheters are disposable respiratory devices designed to safely remove secretions from the respiratory tract of ventilator-dependent patients. The device allows ventilation to continue during suctioning, helps prevent cross-contamination, and protects caregivers from airborne ...
Manufactured by:InterTech Development Company based inSkokie, ILLINOIS (USA)
InterTech provides total test solutions for testing various types of catheters. For instance, multi-lumen catheters are sequentially leak and flow tested at a fast speed using InterTech MED75y two independent channel test instrument. Each lumen of the catheter is either leak or flow tested under 2.0 seconds. ...
Manufactured by:Canadian Hospital Specialties Limited (CHS) based inOakville, ONTARIO (CANADA)
Designed to prevent inadvertent removal or displacement of feeding tubes in adult patients, CORGRIP* Nasogastric/Nasointestinal Tube Retention System is indicated for use with enteral feeding tubes of 8 FR and greater and NG decompression, suction and drainage tubes up to 18 FR. Use of the CORGRIP* system can increase a patient’s caloric intake and may help to avoid unnecessary escalation ...